Question
Question: State Hess’s law of constant heat summation and explain it with an example....
State Hess’s law of constant heat summation and explain it with an example.
Solution
Hint: Before attempting this question, prior knowledge of Enthalpy is a must. Enthalpy is a thermodynamic quantity,equal to the sum of internal energy of the system and the product of pressure and volume (H=U+pV).
Complete step by step solution:
Hess’s law of constant heat summation-
According to this law, the change in enthalpy for a reaction is always the same and does not depend on whether the reaction occurs in one step or multiple steps.
OR
The Hess’s law states that the total enthalpy change during a complete chemical reaction is the same regardless of the path taken by the chemical reaction.
OR
This law states that, the change in enthalpy in a chemical reaction, at a constant pressure is not dependent on the process, and only dependent on the initial and final states of the chemical reaction.
It can be more easily understood by the following diagram.
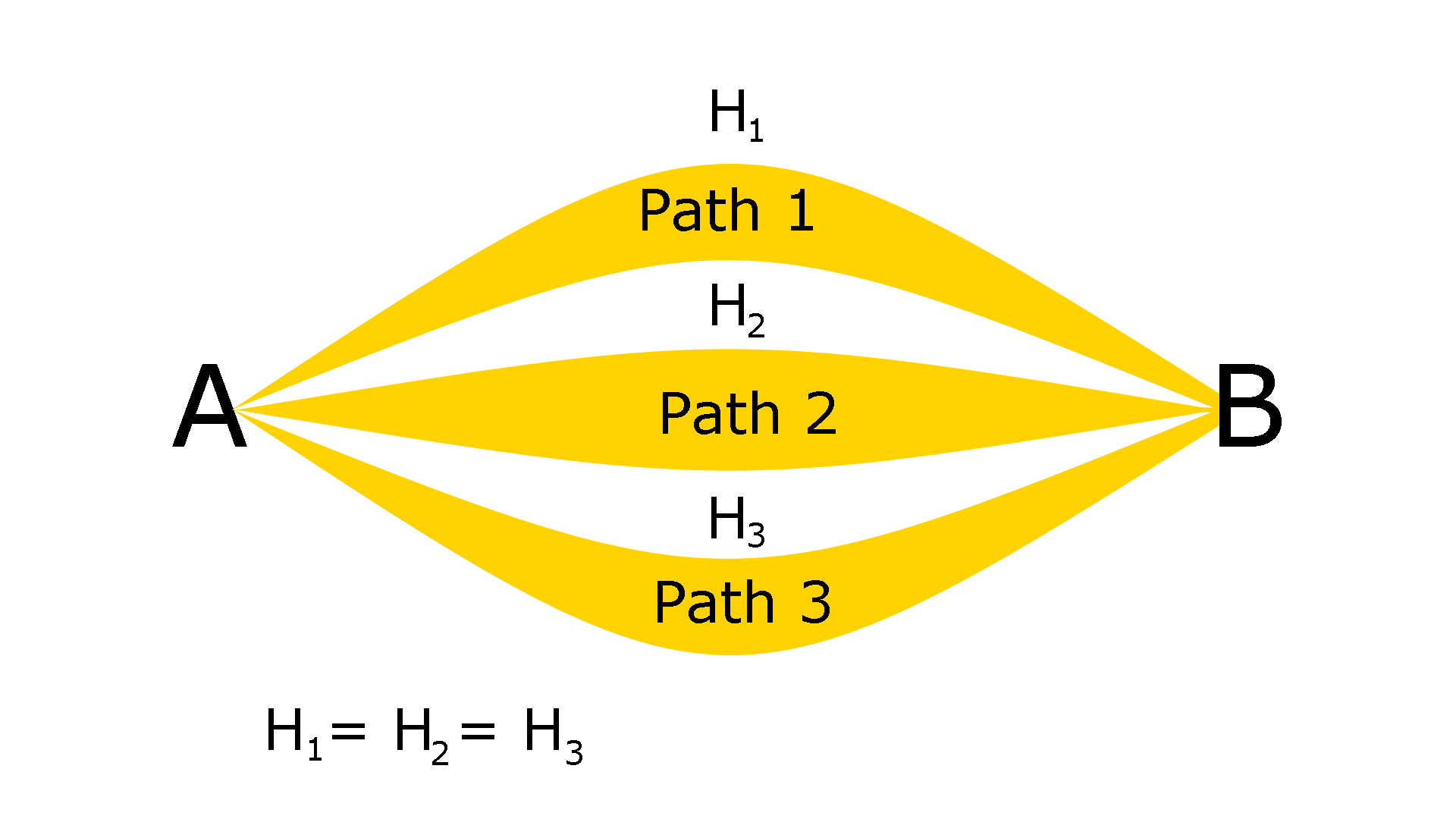
Hess’s law can be seen as an application of the principle of conservation of energy.
As enthalpy only depends upon the initial and final state of the chemical reaction, it is also considered as a state function.
Example:-
Consider the following two routes for preparation of methylene chloride(CH2Cl2) from the reaction between methane(CH4) and chlorine(Cl2)
Route I:
CH4(g)+2Cl2(g)→CH2Cl2(g)+2HCl(g),ΔH10=−202.3kJ
Route II:
CH4(g)+Cl2(g)→CH3Cl(g)+HCl(g),ΔH20=−98.3kJ CH3Cl(g)+Cl2(g)→CH2Cl2(g)+HCl(g),ΔH32=−104.0kJ
Adding change in enthalpy of both steps
CH4(g)+2Cl2(g)→CH2Cl2(g)+2HCl(g),ΔH30=−202.3kJ
Thus, it can be clearly seen that no matter what path we follow, the total enthalpy change in the reaction is always the same.
ΔH10=ΔH20+ΔH32=−202.3kJ
Note: Hess’s law is named after Germain Hess, a Russian chemist who was born in Switzerland. Germain’s Hess’s law of constant heat summation was published in 1840, which is before 1850, when Rudolf Clausius proposed the first law of thermodynamics.
