Question
Question: State Avogadro’s Law....
State Avogadro’s Law.
Solution
A relation between number of moles and volume at constant temperature and pressure is given by V = K n, where ‘n’ is the number of moles of the gas and V is the volume of the gas.
Complete answer:
Avogadro’s law (volume-amount relationship)
This law was given by an Italian Scientist Amedeo Avogadro in the year 1811. He put forward a relation between the volume and number of molecules at constant temperature and pressure. It was experimentally found that the volume of a gas is directly proportional to the number of moles keeping temperature and pressure constant.I.e. V ∝ n (T and P constant)
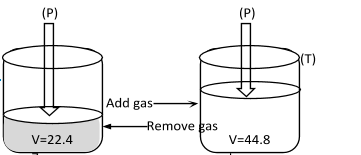
Figure showing V ∝ n at T, P constant.
Where ‘n’ is the number of moles of the gas.
Or V = Kn
Or nV=K
Where K is proportionality constant, now if two gases with volumes V1 and V2 and moles n1 and n2 at constant temperature and pressure are taken then n1V1=n2V2=K
It is evident from the above relation with if the volume of two gases is equal then number of moles is also equal
I.e. if V1 = V2
Then n1 is also equal to n2 (n1 = n2)
In this way Avogadro’s law states that the equal volume of all gases under the same condition of temperature and pressure contain equal number of moles or molecules
Note: As the ideal gas equation expresses the quantitative relation between the four variables that describe the state of a gas, therefore it is called the equation of state for gases.
