Question
Question: Standard electrode potentials for a few half cells are mentioned below : $E_{Cu^{2+}/Cu}^o = 0.34 \ ...
Standard electrode potentials for a few half cells are mentioned below : ECu2+/Cuo=0.34 V,EZn2+/Zno=−0.76 V EAg+/Ago=0.80 V,EMg2+/Mgo=−2.37 V Which one of the following cells gives the most negative value of ΔGo?
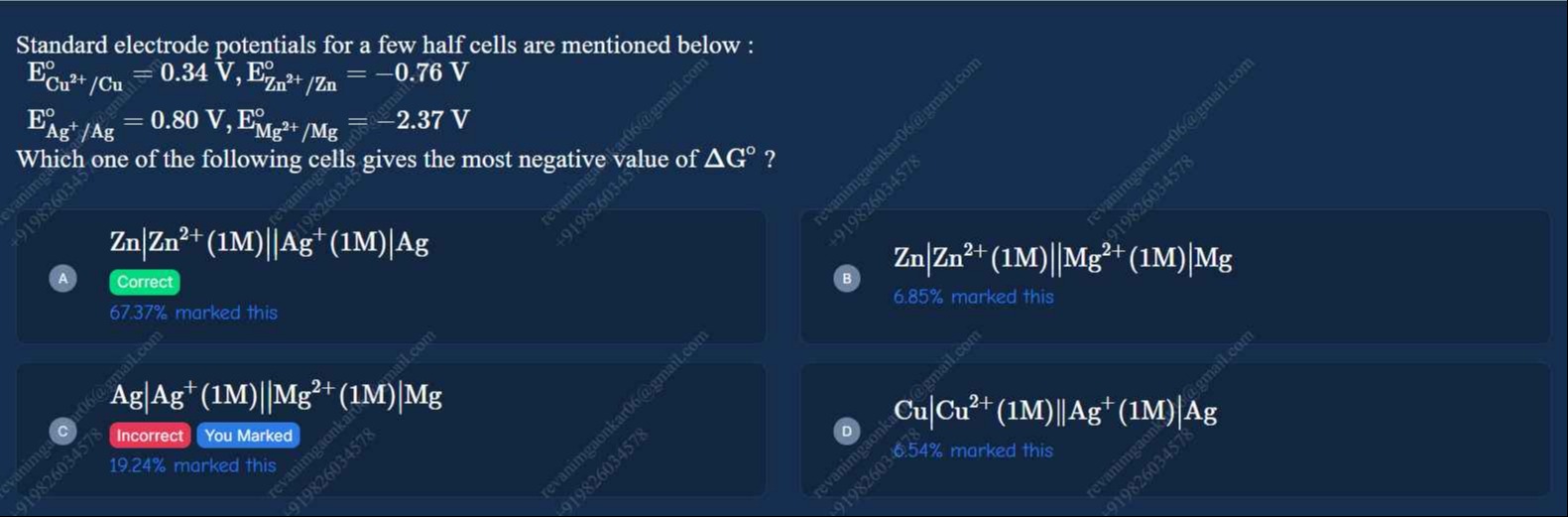
A
Zn∣Zn2+(1M)∣∣Ag+(1M)∣Ag
B
Zn∣Zn2+(1M)∣∣Mg2+(1M)∣Mg
C
Ag∣Ag+(1M)∣∣Mg2+(1M)∣Mg
D
Cu∣Cu2+(1M)∣∣Ag+(1M)∣Ag
Answer
Zn|Zn²⁺(1 M)||Ag⁺(1 M)|Ag
Explanation
Solution
- In a galvanic cell, ΔG° = –nFE°₍cell₎. Thus, a higher positive E°₍cell₎ gives a more negative ΔG°.
- The cell notation convention shows the left electrode as oxidation and the right as reduction. So, E°₍cell₎ = E°₍red (right)₎ – E°₍red (left)₎.
- Option A: Zn|Zn²⁺ (E°₍red₎ = –0.76 V) is oxidized and Ag⁺|Ag (E°₍red₎ = +0.80 V) is reduced.
- E°₍cell₎ = 0.80 V – (–0.76 V) = 1.56 V.
- Higher E°₍cell₎ yields a more negative ΔG°. Options that yield a negative E°₍cell₎ are non‐spontaneous, and the other option (D) gives a lower E°₍cell₎.
