Question
Question: Stable conformer of cis-1-bromo-2-chlorocyclohexane?...
Stable conformer of cis-1-bromo-2-chlorocyclohexane?
Solution
Conformers are isomers obtained by the different spatial arrangements of the single bonds of a molecule. The most stable conformer is the one which is least staggered and is free of torsional strain.
Complete step by step answer:
In the given compound, the bromine and chlorine atoms can take various positions forming a variety of conformers. To find the most stable conformer, we must take a look at each conformer of the chair conformer of cis- 1- bromo- 2- chlorocyclohexane.
Firstly if we consider the case of both the atoms lying in axial positions. This conformer is staggered as both the halide atoms are present on the same side and are held close to each other in this conformation. Also it has a significant torsional strain since both the groups are held outside the plane.
Next, we take into consideration the case of both the halide atoms in the equatorial positions but that too is staggered since both the halide planes are held together in the same plane as that of the ring.
Next we consider taking a bromine atom in an axial position while the chlorine atom in an equatorial position. This conformer is relatively more stable than the previously discussed arrangements as the halide groups are held at a distance from each other and the conformer is less staggered.
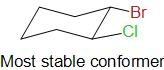
Considering the bromine atom in equatorial position while the chlorine atom in the axial position. This conformer is even more stable than the previous one as it is preferred that the bulkier group be present at the equatorial position as the torsional is higher in the axial position.
Thus, the most stable conformer of cis- 1- bromo- 2- chlorocyclohexane is obtained when the bromine atom is in equatorial position and chlorine is in axial position.
Note:
Cyclohexane ring generally exists as a chair conformer that is almost completely free of strain. Since the cyclohexane ring is planar, any group attached to it can be present at two positions, namely equatorial position or axial position. The bond present at an angle of 90 degrees to the ring plane is known as axial bond. Meanwhile, the bond present in the same plane as that of the ring is known as equatorial bond.
