Question
Question: Stability order of the following species? ...
Stability order of the following species?
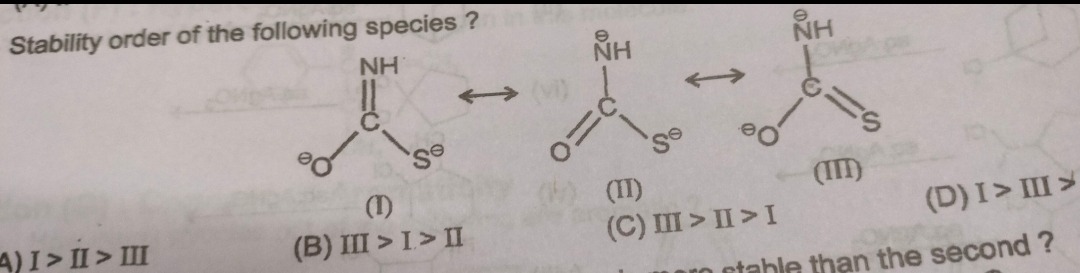
A
I > II > III
B
III > I > II
C
III > II > I
D
I > III >
Answer
I > II > III
Explanation
Solution
The stability of resonance structures is determined by the location of the negative charges. All structures satisfy the octet rule and have the same number of bonds. The order of stability for a negative charge on the involved atoms is S⁻ > O⁻ > N⁻. Structure I places negative charges on O and S, which are the two most stable sites. Structure II places charges on N and S. Structure III places charges on N and O. Since N⁻ is the least stable, Structure I (no N⁻) is most stable. Between II and III, both have N⁻, but S⁻ is more stable than O⁻, making II more stable than III. Thus, the order is I > II > III.
