Question
Question: If the ionization energy of $He^+$ is $19.6 \times 10^{-18}$ J per atom then the energy of $Be^{3+}$...
If the ionization energy of He+ is 19.6×10−18 J per atom then the energy of Be3+ ion in the second stationary state is :
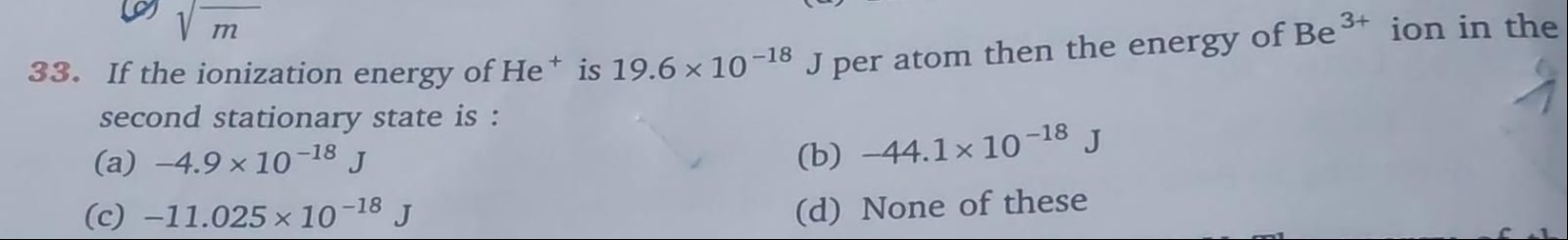
-4.9 x 10^{-18} J
-44.1 x 10^{-18} J
-11.025 x 10^{-18} J
None of these
None of these
Solution
The energy of an electron in the n-th stationary state of a hydrogen-like atom with atomic number Z is given by the formula: En=−kn2Z2 where k is a constant. The ionization energy (IE) is the energy required to remove an electron from the ground state (n=1) to infinity (n=∞), so IE=E∞−E1=0−E1=−E1.
Given the ionization energy of He+ (Z=2) is 19.6×10−18 J. This means the energy of He+ in its ground state (n=1) is E1(He+)=−19.6×10−18 J.
Using the formula for He+ in the ground state (Z=2,n=1): E1(He+)=−k1222=−4k
Equating this to the given ionization energy: −19.6×10−18 J=−4k k=419.6×10−18 J=4.9×10−18 J
Now, we need to find the energy of Be3+ (Z=4) in the second stationary state (n=2). Using the same formula with the calculated constant k: E2(Be3+)=−kn2ZBe2 E2(Be3+)=−(4.9×10−18 J)2242 E2(Be3+)=−(4.9×10−18 J)416 E2(Be3+)=−(4.9×10−18 J)×4 E2(Be3+)=−19.6×10−18 J
The calculated energy, −19.6×10−18 J, is not present in options (a), (b), or (c). Therefore, the correct option is (d).
The expression mV at the beginning of the question is irrelevant to the problem.
