Question
Question: Specify the coordination number of Calcium and Fluoride ions in the compound Calcium fluoride \(Ca{F...
Specify the coordination number of Calcium and Fluoride ions in the compound Calcium fluoride CaF2.
Solution
Coordination number of compounds defined as the total number of spheres or particles closest to the other particles in the crystal lattice.
Complete answer:
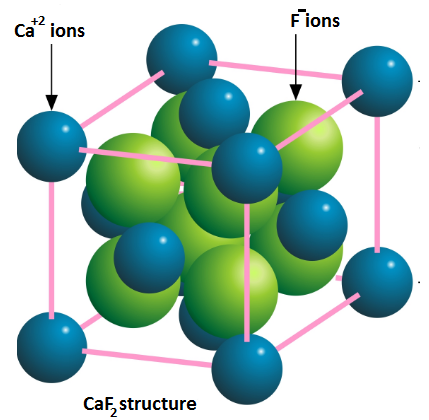
Calcium fluoride is made up of cation of calcium ion Ca+2 and two anions of fluorine ion F−. They collectively form a compound which is represented as AB2 .
These types of structures are also known as Fluoride structure.
In Calcium fluoride compound, Calcium ions Ca+2 has a tendency to present in cubic close packing (ccp). In this arrangement, Calcium ions Ca+2 are surrounded by eight atoms of fluorine ion F−.
While packing into the (ccp) arrangement one ion of calcium is present on each face of the cube to complete the crystal lattice.
In the (ccp) arrangement, one ion of calcium Ca+2 is surrounded by eight Fluorine ion F− which are present at the tetrahedral corner.
Fluorine ion F− in the Calcium fluoride compound arranged in the tetrahedral arrangement and occupies all the apices of a tetrahedron. Therefore, each atom of F− is surrounded by four Ca+2 ions.
From the above discussion we see that one ion of Ca+2 is surrounded by eight F− ions. Hence coordination number of calcium in calcium fluoride compound is 8.
Similarly, one ion of F− is surrounded by four Ca+2 ions. Hence coordination number of fluorine in calcium fluoride compound is 4.
Note:
Sodium oxide is arranged in the same constituent AB2 but it is arranged in the opposite manner of calcium fluoride therefore it is known as anti fluoride structure.
The compound packed as (ccp) arrangement are alphabetically represented as ABCABCA....
