Question
Question: Solubility of Mg(OH)₂ Is maximum In [Ksp of Mg(OH)₂ = 4 × 10⁻³]...
Solubility of Mg(OH)₂ Is maximum In [Ksp of Mg(OH)₂ = 4 × 10⁻³]
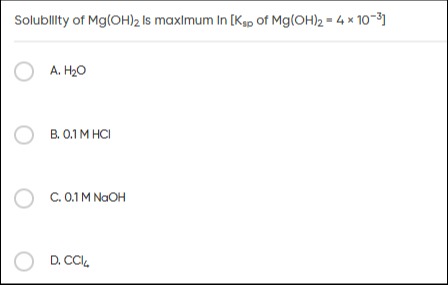
A
H₂O
B
0.1 M HCl
C
0.1 M NaOH
D
CCl₄
Answer
0.1 M HCl
Explanation
Solution
Mg(OH)₂ dissociates as Mg(OH)₂(s) <=> Mg²⁺(aq) + 2OH⁻(aq). In acidic solutions, H⁺ ions react with OH⁻ ions, reducing their concentration and shifting the equilibrium to the right, thus increasing solubility. HCl is an acid, which will significantly increase the solubility of Mg(OH)₂ by consuming OH⁻ ions. NaOH is a base and would decrease solubility due to the common ion effect (presence of OH⁻). H₂O provides a neutral medium. CCl₄ is a non-polar solvent and will not dissolve ionic Mg(OH)₂ effectively. Therefore, solubility is maximum in 0.1 M HCl.
