Question
Question: Solder is used for joining two metal surfaces together. Solder is a mixture of lead and tin. Can two...
Solder is used for joining two metal surfaces together. Solder is a mixture of lead and tin. Can two metals be separated by distillation?
Solution
Hint:
Distillation is a separation technique that can be used to either increase the concentration of a particular component in the mixture or to obtain (almost) pure components from the mixture. With this in mind, try to work out whether this process could be used in the separation of metals.
Step-by-Step Solution:
Let us first understand the concepts of metal refinery and distillation separately before moving onto analysing whether there can be any overlap between the two.
Refining is a method of removing impurities in order to obtain metals of high purity. The impurities are removed from crude metal by various methods based on the properties of the metal and the properties of impurities.
It is to be distinguished from other processes such as smelting and calcining in that those two involve a chemical change to the raw material, whereas in refining, the final material is usually identical chemically to the original one, only it is purer. The processes used are of many types, including pyrometallurgical and hydrometallurgical techniques.
The process of distillation exploits the difference in the boiling points of the components in the liquid mixture by forcing one of them into a gaseous state. It is important to note that distillation is not a chemical reaction but it can be considered as a physical separation process.
Now, let us try and understand if two metals can possibly be separated by the process of distillation.
Distillation of metals is used for the purification of metals which possess a low boiling point such as mercury and zinc. In this process the impure metal is heated above its boiling point so that it can form vapours. The impurities do not vaporize and hence they are separated. The vapours of the pure metal are then condensed leaving the impurities behind.
An illustration of the same is as follows:
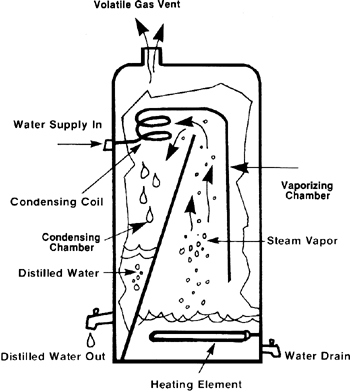
Note:
For metals that have a particularly low boiling point, such as mercury and zinc, they can be distilled to remove the impurities. So, if you heat up a sample of impure mercury, the mercury will vaporize but nothing else around it will. The vapor can be collected, and when it condenses back you are left with pure mercury.
