Question
Question: Sodium hypohalite when dissolved in water will form (A) Hypohalous acid (B) Hydrochloric acid ...
Sodium hypohalite when dissolved in water will form
(A) Hypohalous acid
(B) Hydrochloric acid
(C) Sodium chloride
(D) None of these
Solution
Hypohalite is an oxyanion bearing a halogen in oxidation state +1 . Sodium hypohalite is used in the haloform reaction (chemical reaction in which haloform is produced by the exhaustive halogenation of a methyl ketone in the presence of a base). Sodium hypohalite in this reaction is used as a test for methyl ketones.
Complete answer:
Sodium Hypochlorite is a slightly yellowish solution with a characteristic odor and is used as disinfectant or a bleaching agent. Sodium Hypochlorite comprises a sodium cation (Na+) and a Hypochlorite anion (ClO−) . It is the sodium salt of hypochlorous acid.
When sodium Hypochlorite is dissolved in water it dissociates to form Hypochlorous acid (HOCl)
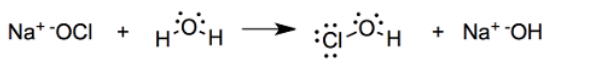
Sodium Hypochlorite is a salt of a moderately strong base (OCl)− and a weak acid (HOCl)
NaOCl+H2O→Na++OCl−
OCl−+H2O→HOCl+OH−
Therefore the correct answer is option A. Sodium Hypochlorite when dissolved in water will form Hypohalous acid (Hypochlorous acid).
Note:
The acidic strength of oxoacids having the same oxidation number of the halogen atom decreases with increase in the atomic number i.e., with decreasing electronegativity of the atom and
with the increase in the oxidation number of a particular halogen atom, the acidic character of corresponding oxoacid increases. This can be explained on the basis of the Lowry Bronsted concept. According to this concept, a strong acid has a weak conjugate base and a weak acid has a strong conjugate base.
Among the halogens, fluorine has very little tendency to form oxoacids due to its small size and high electronegativity. However, it forms one oxoacid HOF .
