Question
Question: Sodium format on heating yields A.Oxalic acid and \[{H_2}\] B.Sodium oxalate and \[{H_2}\] C...
Sodium format on heating yields
A.Oxalic acid and H2
B.Sodium oxalate and H2
C. CO2 and NaOH
D.Sodium oxalate
Solution
Sodium format (HCOONa) is an organic sodium salt which is the monosodium salt of formic acid. It plays the role of a buffer and as well as an astringent. Sodium format contains a format. When we heat Sodium format to a temperature between 220-360 degree Celsius, decomposition reaction takes place.
Complete step by step answer:
Sodium format or HCOONa, is the sodium salt of formic acid HCOOH. It usually appears as a white deliquescent powder. On heating of sodium format, decomposition reaction occurs. As a result of this reaction sodium oxalate is formed and hydrogen is released.
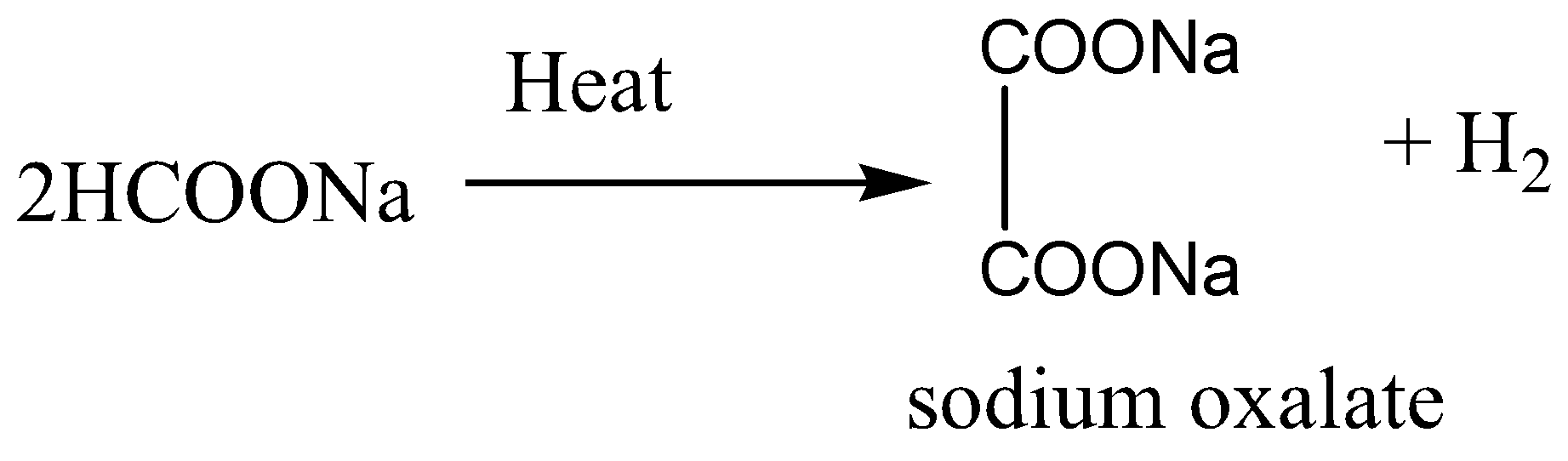
When sodium formate is heated at 220−3600C, a melt is formed, for getting Sodium oxalate, which is then spread thinly as a liquid over hot surfaces heated to a temperature between 360−4400C, where the decomposition to sodium oxalate takes place in 2 to 3 seconds with a quantitative yield conversion of format to oxalate.
If sodium formate is heated rapidly at 400 to 4200C, the reaction time of thermal decomposition is much reduced and in case this optimum temperature is maintained, there is maximum production of sodium oxalate.
Therefore, the correct answer is option (B).
Note: Sodium formate is heated rapidly at 400 to 4200C that is overheating of sodium format in order to maximize the product, sodium oxalate (further decomposition of sodium oxalate), as possible to reduce the reaction time with optimum temperature of thermal decomposition. Overheating may cause decomposition of sodium oxalate.
Sodium oxalate is an organic sodium salt which consists of sodium ions and oxalate ions in a ratio of 2:1. It plays the role of a poison as well as a reducing agent. It is an oxalate salt and an organic sodium salt and contains an oxalate (2−).
