Question
Question: Sodium ethoxide has reacted with ethanoyl chloride. The compound that is produced in above reaction ...
Sodium ethoxide has reacted with ethanoyl chloride. The compound that is produced in above reaction is:
A) Ethyl chloride
B) Ethyl ethanoate
C) Diethyl ether
D) 2- Butanone
Solution
When a chemical species donates its electron to form a chemical bond then it is called a nucleophile. A molecule having lone pair or at least one pi bond shows nucleophilic behaviour. Nucleophilic character is used to compare the affinity of atoms.
Complete step-by-step answer:
Sodium ethoxide is defined as an organic compound having a chemical formula C2H5ONa. It gradually changes its colour dark when stored in dry air because of oxidation. It also acts as a strong base and shows corrosive behaviour.
Ethanoyl chloride is an acetyl chloride derived from acetic acid. It does not exist in nature when contact with water results in the formation of acetic acid and hydrogen chloride.
When sodium ethoxide is reacted with ethanoyl chloride it results in the formation of an ester compound that is ethyl ethanoate. In this reaction, C2H5O− ,this is a strong nucleophile attacks on a carbonyl complex having slightly positive charge on carbon and a slightly negative charge is formed on oxygen.
The oxygen will not sustain the negative charge and form a double bond and replaces the weak nucleophile that chlorine. This results in the formation of ethyl ethanoate.
Let us see the chemical reaction:
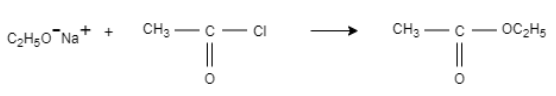
The compound produced due to this reaction is ethyl ethanoate.
Hence, the correct answer is option ‘B’.
Note: When a hydroxyl group is replaced by an alkoxy group, then an ester is formed. It is also derived from carboxylic acid. Esters are carbon to oxygen double bonds that are singly bonded to a second oxygen atom. It is a sweet smelling compound. Nucleophiles are the anions having negative charge. Ambident nucleophiles are compounds that attack from two or more places.
