Question
Question: Sodium acetate on heating with soda lime produce: (A) \(C{{H}_{4}}\) (B) \({{C}_{2}}{{H}_{6}}\) ...
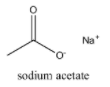
Sodium acetate on heating with soda lime produce:
(A) CH4
(B) C2H6
(C) C3H8
(D) C2H4
Solution
Sodium salt of carboxylic acid on heating with soda lime produces alkane with one less carbon atom than carboxylic acid. Soda-lime is a mixture of sodium hydroxide and calcium oxide. Acetic acid has two carbon atoms. It is a decarboxylation reaction as there is the elimination of carbon dioxide from a carboxylic acid.
Complete step by step solution:
-Decarboxylation is a method to prepare alkane using sodium salt of carboxylic acid.
-This method can be used when one less carbon atom than carboxylic acid is required or when the number of carbon atoms is to be reduced by one.
-When sodium salt of the carboxylic acid is treated with soda lime which is a mixture of sodium hydroxide and calcium oxide, carbon dioxide is eliminated so an alkane is produced with one less carbon atom than carboxylic acid and sodium bicarbonate is produced as a by-product.
-Calcium oxide is used so Sodium hydroxide can be easily handled. Sodium hydroxide is highly hygroscopic and easily forms concentrated sodium hydroxide when exposed to air. Soda-lime does not absorb moisture easily.
-Soda-lime has the ability to absorb carbon dioxide. When carbon dioxide is eliminated from carboxylic acid, it reacts with sodium hydroxide to form sodium carbonate.
CH3COONa+NaOHCaO,ΔCH4+Na2CO3
When sodium acetate on heating with soda lime, methane is produced.
Sodium acetate on heating with soda lime produce: CH4, which is option (A)
Note: In decarboxylation, the sodium salt of carboxylic acid on heating with soda lime produces alkane with one less carbon atom than a carboxylic acid. In decarboxylation, the product is alkane having one less carbon atom than a carboxylic acid. If ethane is to be prepared, then sodium propionate should be used. IF carboxylic acid has n number of carbon atoms, then alkane produced will have (n-1) carbon atoms.
