Question
Question: Six moles of an ideal gas performs a cycle shown in figure. The temperatures are \[{T_A} = 600K,{T_B...
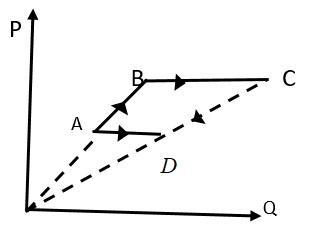
Six moles of an ideal gas performs a cycle shown in figure. The temperatures are TA=600K,TB=800K,TC=2200KandTD=1200K. The work done by the cycle ABCDA is
A 20KJ B30KJ C40KJ D60KJ
Solution
As we know that we have to find out total work done by the cycleABCDA , to calculate this we need to find work done by AB,BC,CDandDA. Then add all of them, and we will get work done by cycle.
Formula used: ΔWBC=nRΔT
Given: Moles of gas, n=6and Temperatures as below:
TA=600K
TB=800K
TC=2200K
TD=1200K
Complete step by step solution:
When volume of a gas remains constant this is known as an isochoric process which is shown by AB and CD given in figure.
ABis isochoric process,
So, work done in ABwill be zero
ΔWBC=0......(1)
When pressure of a gas remains constant then the process will be an isobaric process as shown by BC and DA in the given figure.
BC is isobaric process,
So, work done for ideal gas in BCwill be
ΔWBC=nRΔT (Here R is universal gas constant)
=nR(TC−TB)
Putting values ofTc , TB and n
=6×R(2200−800)
=8400R
Here we will put value of universal gas constant, R=8.3J/K−mol
=8400×8.3=69720J......(2)
CDis isochoric process,
So, work done in CDwill be zero
ΔWCD=0......(3)
DA is isobaric process,
So, work done for ideal gas in DCwill be
ΔWDA=nRΔT
=nR(TA−TD)
Putting values of Tc , TB and n
=6×R(600−1200)
=6×R×(−600)
=−3600R
Here we will put value of universal gas constant, R=8.3J/K−mol
=−3600×8.3
=−29880J......(4)
Now, we will add all four work done to find total work done by the cycleABCDA
ΔWTotal=ΔWAB+ΔWBC+ΔWCD+ΔWDA......(5)
=0+69720+0−29880(Putting values of equation 1, 2, 3 and 4 in equation 5)
=39840J
Here, the total work done by cycleABCDA.
Note: We should note that, to find out work done by a cycle, we must break that cycle in individual parts. And calculate their individual work done with the help of the formula of work done, then add all of them.
