Question
Question: Similar to alkenes and alkynes benzene also undergoes ozonolysis. In the sequence of the given react...
Similar to alkenes and alkynes benzene also undergoes ozonolysis. In the sequence of the given reaction identify X and Y
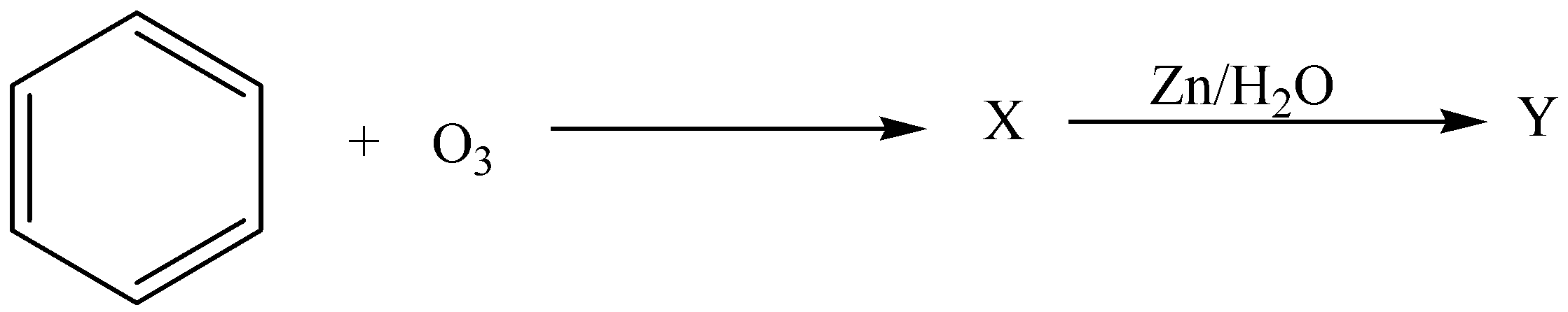
A.X=Triozonide,Y=Glyoxal
B. X=Diozonide,Y=Succinicacid
C. X=molozonide,Y=BenzoicAcid
D. X=Triozonide,Y=Benzaldehyde
Solution
We have to know that the benzene, is a simplest hydrocarbon (contains only carbon and oxygen) having the molecular formula is C6H6 and it is a cyclic planar ring. Benzene word comes from benzoin resin. Benzene is one of the elementary petrochemical constituents and also a natural constituent of crude oil.
Complete answer:
We know that the Ozone (also known as trioxygen) is an allotrope of oxygen, a triatomic molecule, molecular formula is O3 . Chris Friedrich Schonbein invented the ozonolysis in 1840 .
We need to know that the Ozonolysis involves the addition of ozone molecules across the unsaturated double bond of alkene, alkynes or azo compounds, are cleaved. The carbon-carbon double bond has been replaced by a carbonyl group in the ozone. And then the ozonide is cleaved to form smaller fragments.
In the given reaction,
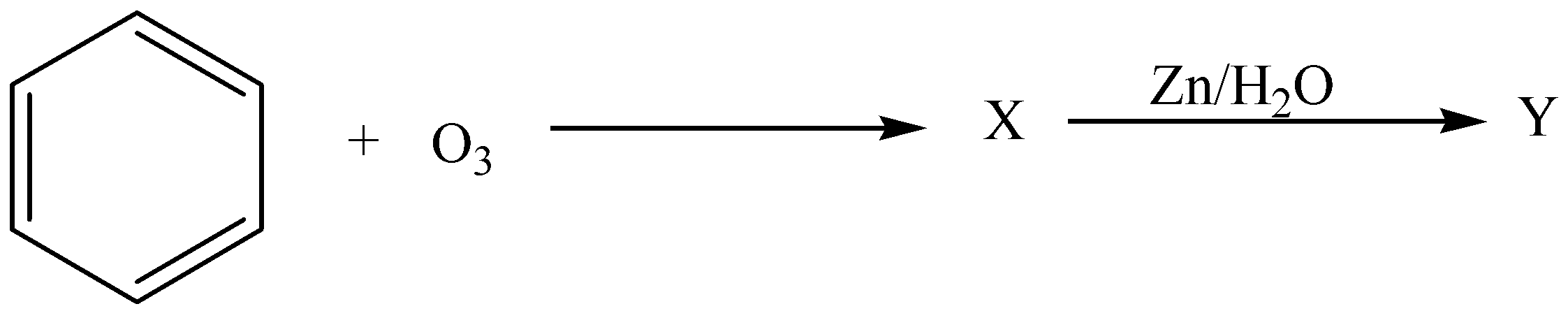
The X and Y are,
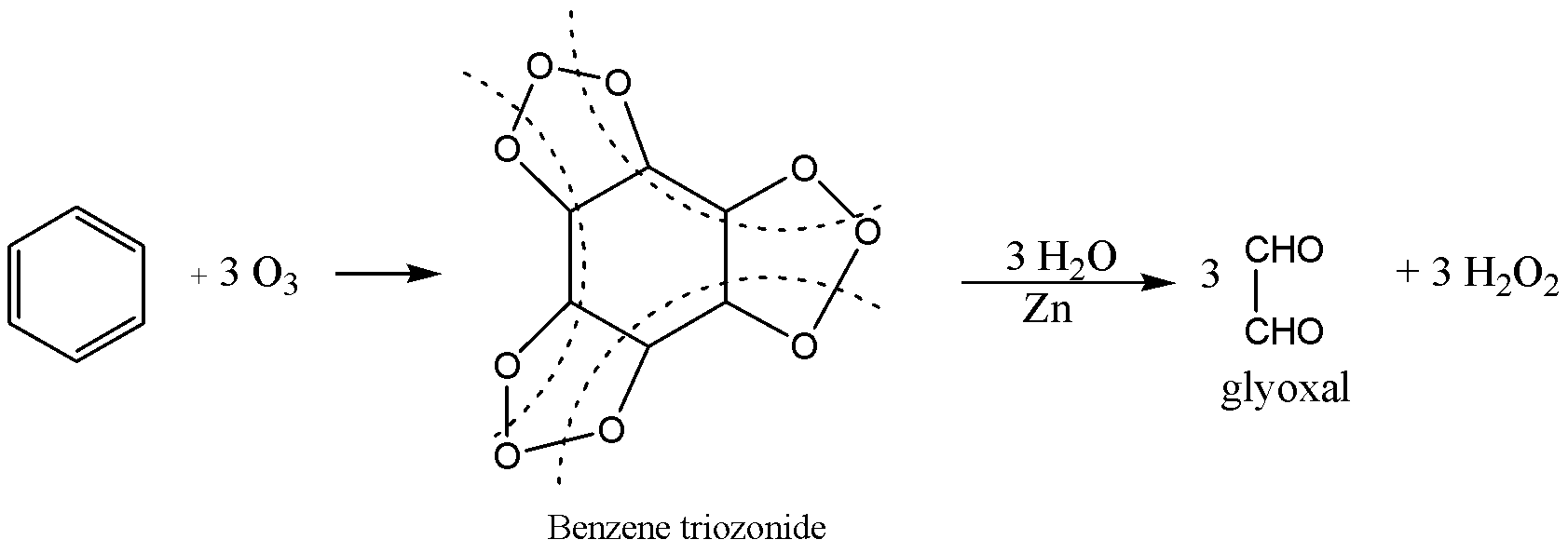
Ozone is a resonance hybrid structure of several structures and in one of the structures, the terminal oxygen acts as electrophile, the electrophile adds to benzene to form benzene triozonide then reduction to give glyoxal and peroxide.
From the above reaction X and Y are benzene triozonide and glyoxal.
The correct option is A. X=Triozonide,Y=Glyoxal .
Note:
We have to know that the Ozonolysis is an important reaction for locating a double bond in the compound. In Ozonolysis, the end products formed are given useful information about the structural environment of the C=C . Carl Dietrich Harries was some attribute to this ozonolysis, so alkene ozonolysis sometimes referred as Harries Ozonolysis. Ozonolysis is particularly used in natural product synthesis, and also in organic chemistry, industrial-scale synthesis of pharmaceuticals.
