Question
Question: Silicon’s are synthetic polymers containing repeated \({{R}_{2}}SiO\) units. (A) True (B) False...
Silicon’s are synthetic polymers containing repeated R2SiO units.
(A) True
(B) False
Solution
Polymers are macromolecules built up by linking together a large number of small molecules. The repeating units in a polymer are linked through strong covalent bonds. Small molecules combined to form polymer molecules are called monomers. Carbon and silicon are from group 14 in the periodic table that silicon also forms polymers like carbon because of its catenation property.
Complete step by step solution:
Generally, polymers are classified into many types based on their physical and chemical properties.
The classification of polymers is:
-Based on source
-Based on polymerization
-Based on functionality
-Based on intermolecular forces
-Based on monomers
-Based on the source:
Polymers are classified into two types based on availability of source or abundance in nature. Those are natural polymers and synthetic polymers.
Natural polymers: those polymers are available in nature such as plants and animals.
Examples: proteins, cellulose, starch, some resins, and natural rubber.
Synthetic polymers: These varieties of synthetic polymers as plastics, synthetic fibers, and synthetic rubbers, silicon’s are examples of man-made polymers prepared in daily life as well as in industry or laboratories.
Silicones:
These polymers are known as synthetic organ silicon polymers, which contain repeated R2SiO units by Si-O-Si linkages.
Silicones compounds have a general formula (R2SiO)n, where R = alkyl or aryl group.
Polymerization of Silicones:
Step-1: when silicon reacts with methyl chloride in presence of copper catalysts at 570K, various types of methyl-substituted chlorosilanes CH3SiCl3,(CH3)2SiCl2,(CH3)3SiCl,(CH3)4Si are formed.
Step-2: hydrolysis (CH3)2SiCl2 is followed by polymerization yields chain polymer.
(CH3)2SiCl2+H2O→(CH3)2Si(OH)2+2HCl
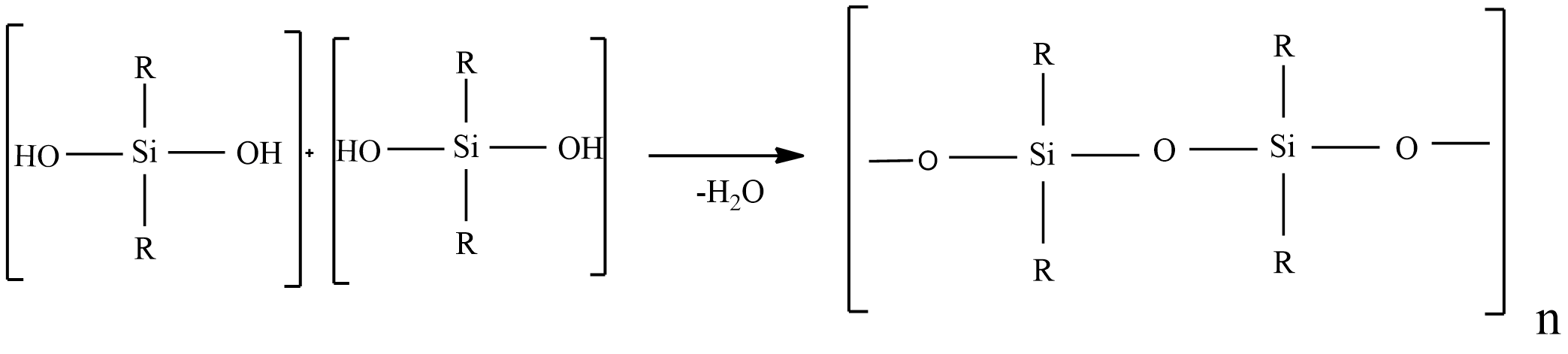
So, the given statement Silicon’s are synthetic polymers containing repeated R2SiO units is true.
The correct option is A.
Note: Silicones are made up of short-chain molecules that are oily liquid, medium chains behave as viscous liquids, greases and very long chains behave as elastomers and resins. These are chemically inert and easily attacked by organic reagents. These are heat resistant and have a high dielectric strength.
