Question
Question: \(SiH_{3})_{3}N\) is: A.Planar B.Pyramidal C.Octahedral D.Tetrahedral...
SiH3)3N is:
A.Planar
B.Pyramidal
C.Octahedral
D.Tetrahedral
Solution
Hint:**To obtain the solution of the question, we need to refer to the valence bond theory which explains the structure and magnetic properties of coordination compounds.
Complete step-by-step answer: - The compound given in question, has pπ − pπ bond present. From the molecular formula we get to know that the concept of back bonding is followed here.
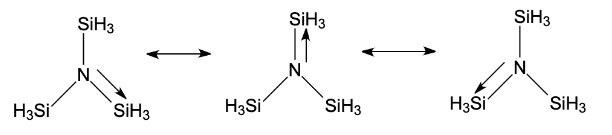
- From the structure of the given compound, we know that silicon has vacant d-orbitals. The lone pair on N provides vacant d-orbitals of Silicon with the required lone pair of electrons. Hence, N−SiH3 bond achieves a partial double bond character that is, its hybridization changes and becomes sp2which refers to the trigonal planar shape of the molecule.
- Now, with reference to the concept of back bonding as a sort of resonance between lone pair of electrons and the other atom with a vacant d or p-orbital present in their central atom sulfur. Hence, gives a planar shape.
Therefore, the correct option is (A).
Note: Valence bond theory tells us that the metal ion or atom under the influence of ligands uses (n-1) d, ns, np, and d orbitals for hybridization. This theory yields a set of equivalent orbitals that define geometry in terms of octahedral, tetrahedral, square planar etc. These hybridized orbitals can also overlap with the ligand orbitals to donate electron pairs for bonding.
