Question
Question: Show the formation of \[MgO\] by transfer of electrons between combining atoms....
Show the formation of MgO by transfer of electrons between combining atoms.
Solution
Magnesium oxide is an inorganic compound that occurs in nature as the mineral periclase. Aqueous media combines quickly with water to form magnesium hydroxide. It is used as an antacid and mild laxative and has many non medicinal uses.
Complete step by step answer:
The atomic number of Magnesium is12.
The electronic configuration of magnesium is
Mg(Z=12) = 1s22s22p63s2
In order to obtain an octet configuration, it will lose two electrons.
The atomic number of oxygen is8.
The electronic configuration of oxygen is
O(Z = 8) = 1s22s22p4
In order to obtain an octet configuration, it has to gain two electrons.
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing two electrons, the magnesium atoms form a magnesium ion (Mg2+) and by gaining two electrons, the oxygen atom forms an oxide ion (O2−).
Mg: + O → MgO
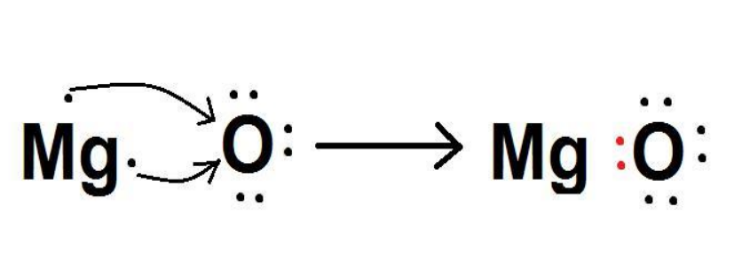
Note: Magnesium oxide nanoparticles can be prepared using the hydroxide precipitation process, which is followed by the thermal decomposition of the hydroxide. MgO Can be characterized by X-ray powder diffraction and scanning electron microscopes.
Magnesium oxide nanoparticles can be applied in electronics, catalysis, ceramics, petrochemical products, coatings, and many other fields. Magnesium oxide nanoparticles can be used along with wood chips and shavings to make materials such as sound-proof, light-weight, heat-insulating, and refractory fiberboard and metallic ceramics.
Magnesia (magnesium oxide,MgO) is mainly produced from the calcination of magnesite in a process similar to the production of lime from limestone. A smaller proportion of the world's MgO production comes from seawater and brine sources.
