Question
Question: Show the electron dot representation of (A) \( LiCl \) (B) \( CaO \)...
Show the electron dot representation of
(A) LiCl
(B) CaO
Solution
We know that the electron dot representation such that the dot shows the valence electrons of the atom. This representation of dots surrounding the atom is known as the electron dot representation of that atom. First of all we have to study the valence and the electronic configuration of that atom and represent that valence electron in the form of dots surrounding that atom.
Complete answer:
(A) LiCl
Here the compound given is LiCl , for the electron dot configuration or representation we must know about these atoms electronic configuration as:
Electronic configuration of Li : 1s22s1
Electronic configuration of Cl : 1s22s22p63s23p5
Valence electron of Li is 1 and of Cl is 1
Therefore, the electron dot representation is given in the following figure as:
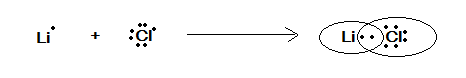
In the above figure we have represented the electron dot representation of LiCl where these two atoms share a pair of electrons. They both satisfy the valence of each other.
(B) CaO
Here, the compound given is CaO . So we have to first write the electronic configuration as we have written in the above solution. Thus,
Electronic configuration of Ca : 1s22s22p63s23p64s2
Electronic configuration of O : 1s22s22p4
Valence electron for Ca is 2 and of O is 6
Therefore, the electron dot representation is given in the following figure as:
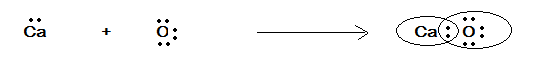
In the above figure we have the electron dot representation of CaO where these two atoms share a pair of electrons. They both satisfy the valence of each other.
These are the electron dot representation of the given compounds.
Note:
Here, it is necessary to know the configuration of the required element and the valence electrons are represented in the electron dot structure of the compounds as we have done above. We must take care of the valence electrons that means the electrons that are in the outermost shell and here these compounds satisfy each other’s valency by sharing electrons each possess.
