Question
Question: Show how you will synthesise: (i) 1-Phenylethanol from a suitable alkene. (ii) Cyclohexylmethano...
Show how you will synthesise:
(i) 1-Phenylethanol from a suitable alkene.
(ii) Cyclohexylmethanol using an alkyl halide by a SN2reaction.
(iii) Pentan-1-ol using a suitable alkyl halide.
Solution
These reactions are either electrophilic addition or nucleophilic substitution reaction. In an electrophilic addition reaction, a π bond is broken and two new σ bonds are formed. In nucleophilic substitution reaction, a leaving group is replaced by an electron rich compound.
Complete step by step solution:
Synthesis of the following reaction:
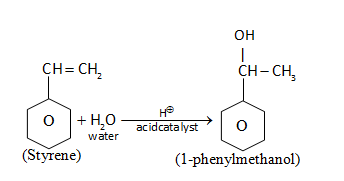
(i) 1-phenylethanol from suitable alkene.
Alkene is treated with H2Oin the presence of acid (acid catalyzed hydrolysis) to give 1-phenylmethanol
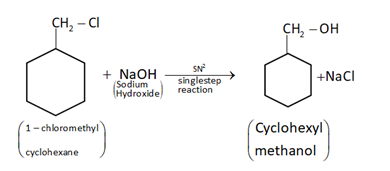
(ii) Cyclohexylmethanol using an alkyl halide by a SN2 reaction.
When chloromethylcyclohexane is treated with sodium hydroxide, then it will give cyclohexylmethanol by SN2mechanism.
(iii) Pentan-1-ol using a suitable alkyl halide.
When chloropentane is treated with aq.NaOH, then it will give pentan-1-ol.
