Question
Question: Show how could you accomplish the following synthesis? Cyclohexyl amine \(\longrightarrow\) N- cyc...
Show how could you accomplish the following synthesis?
Cyclohexyl amine ⟶ N- cyclohexyl acetamide
Solution
Cyclohexylamine is a cyclic, aromatic structure with 6 carbon and an amine group attached to it. For the conversion of amine to the amide group, the hydrogen is removed from the amine and carbonyl group (C=O) attaches in place of hydrogen and forms acetamide group i.e. NH3-CO-NH3 .
Complete answer:
-To convert the cyclohexylamine let's draw the structure first.
-As it has 6- membered cyclic ring with an amine group so the structure is:
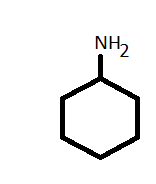
-Now, in N - cyclohexyl acetamide has a similar structure to cyclohexylamine except the presence of acetamide group in place of the amine group.
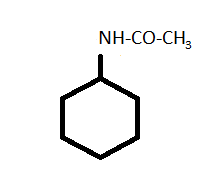
-For the synthesis of N - cyclohexyl acetamide from cyclohexylamine, pyridine is used as a catalyst to increase the rate or speed of the reaction.
-Along with the pyridine, acetyl chloride is used which has a molecular formula of CH3-CO-Cl.
-From this, the oxidation of cyclohexylamine takes place due to which hydrogen atom releases from -NH2 the group.
-Meanwhile, from acetyl chloride chlorine is released.
-So, the CH3 - CO- group attaches with the amine group of the cyclohexylamine and it forms cyclohexyl acetamide.
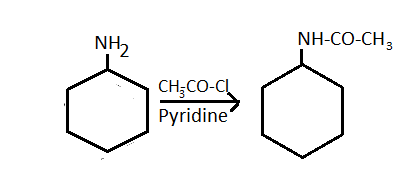
Cyclohexylamine N - cyclohexyl acetamide
-Along with it, hydrochloric acid is also formed.
-So, by using acetyl chloride we can synthesize N - cyclohexyl acetamide.
Note: It is observed that when N - cyclohexyl acetamide is reduced by LiAlH4 which is a strong reducing agent, then the oxygen is lost from the acetamide and it can form N - ethyl cyclohexylamine, which has extra alkyl group than cyclohexylamine.
.
