Question
Question: Shape of \(XeO{F_4}\) is: A. Octahedral B. Square pyramidal C. Pyramidal D. T-shaped...
Shape of XeOF4 is:
A. Octahedral
B. Square pyramidal
C. Pyramidal
D. T-shaped
Solution
At first, the hybridisation state of the XeOF4 molecule needs to be determined. This state of hybridisation will determine the shape of the given molecule and hence the shape will be according to the given configuration of the electrons in the orbitals.
Complete answer:
The shape of the molecule XeOF4 has the electronic configuration in such a manner so that there is a chemistry of square pyramidal. The internal molecule which is the central atom is Xe and there are four F residues which are associated with the central atom. This forms the basal structure of the XeOF4 molecule. The associated O residue forms a double-bonded structure with the Xe residue. Therefore, the internal Xe residue forms six bonds with different molecules. There are 8 valence electrons in the electronic configuration of Xe residue. Therefore as electrons are already forming bonds, there is a lone pair of electrons which is left without any possible bond formation. This lone pair is present as the trans structure associated with Xe residues. The hybridisation is based on the electron filling of the orbitals. The hybridisation of this chemical molecule XeOF4 is sp3d2, which means according to the nature of hybridisation the structure is square pyramidal. The pyramidal structure is because of the O residue which is present on a different plane. The resultant structure of the XeOF4 is given in the diagram.
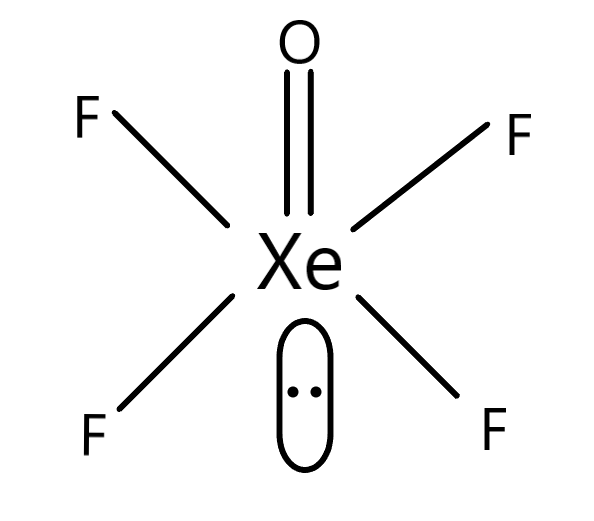
Hence, option B is correct.
Note:
The XeOF4 molecule is a structure which has the super-octet character. This is because as a result of the covalent interactions of all the atoms with the central Xe atom the number of electrons is more than the natural octet filled level. Hence this molecule does not follow the octet rule.
