Question
Question: Shape of P-orbital is: A. Spherical B. dumb-Bell C. Double dumb bell D. None of these...
Shape of P-orbital is:
A. Spherical
B. dumb-Bell
C. Double dumb bell
D. None of these
Solution
To determine the shape of p-orbitals we should know the structure of the p-orbital and the number of lobes present in the p-orbital. P- subshell has three orbits. Three orbital shells are denoted as PX,PY, and PZ.
Complete step-by-step solution: The number of orbitals in a subshell is determined by the following formula:
2l + 1
Where,
l is the azimuthal quantum number.
The l value of p-orbital is 1.
So,
2×1 + 1
3
The P-subshell has three, PX,PYand PZorbitals. All these orbitals are degenerate. All orbitals of the p-subshell lie on the axis. So, the electron density of each orbital also lies on the axis. Each p-orbital has two lobes.
The positions of p-orbitals on the axis is represented as follows:
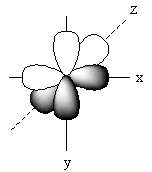
The positions of these three p-orbitals on the axis separately is represented as follows:
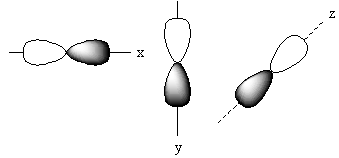
Name of each p-orbital depends upon the axis. The p-orbital which lies on the x-axis is known as Px orbital. The p-orbital which lies on the y-axis is known as PY orbital. The p-orbital which lies on the z-axis is known as PZ orbital.
Each p-orbital has two lobes that are arranged like a dumb bell so, the shape of p–orbital is dumb bell.
So, the shape of P-orbital is dumb-Bell.
Therefore, option (B) dumb-Bell, is correct.
Note: The s-subshell has only one s-orbital. S-orbital has one lobe of spherical shape so, the shape of s-orbital is spherical. D-subshell has five d-orbitals. Out of five d-orbitals, two lie on the axis and three lie in between the axis. dz2and dX2−Y2orbitals which lies on the axis and the dXZ,dYZ, and dXY lies in between the axis. Except dz2all d-orbital has four lobes so, the shape of d-orbital is double dumb bell.
