Question
Question: Shape of p-orbital is: (a)- Spherical (b)- Dumb-bell (c)- Double dumb-bell (d)- None of thes...
Shape of p-orbital is:
(a)- Spherical
(b)- Dumb-bell
(c)- Double dumb-bell
(d)- None of these
Solution
Every orbital has a shape in which the probability of finding the electron is the most. From probability calculations, it is found that in p-orbital the electrons are maximum found in two lobes on the opposite side of the nucleus.
Complete step by step answer:
Every orbital has a shape in which the probability of finding the electron is the most. Based on probability calculations, it is found that the maximum probability of finding the p-electrons is in the two lobes on the opposite sides of the nucleus. So according to this the shape of the p-orbital will be a dumb-bell shape. The shape is given below:

It must be noted that the probability of finding the electron in both the lobes are equal. In the diagram one lobe is shaded and another lobe is non-shaded, which implies, one lobe has a positive charge and the other has a negative charge.
We know that for p-orbital, the azimuthal quantum number is 1, so the magnetic quantum number will be 3 (-1, 0, +1). So it will have three orientations. These are represented as px, py, and pz. Their shapes are given below:
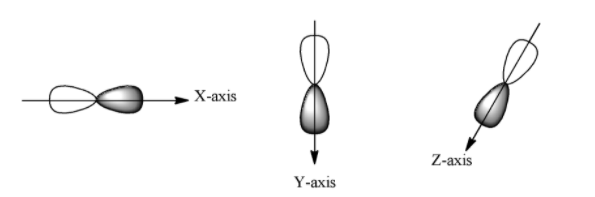
So each lobe has a positive side and a negative side and the p-orbital have directional characteristics.
Therefore, the correct answer is an option (b)- Dumb-bell.
Note: All the orbitals of p are degenerate, i.e., they have the same energies. The center of the lobes has a plane passing through the nucleus where the probability of finding the electron is zero.
