Question
Question: Shape of \[{{\left( \mathbf{Si}{{\mathbf{H}}_{\mathbf{3}}} \right)}_{\mathbf{3}}}\mathbf{N}~\] with ...
Shape of (SiH3)3N with respect to N is:
A.Pyramidal.
B.Tetrahedral.
C.Trigonal Planar.
D.T-Shaped
Solution
For solving these types of questions, firstly we need to refer to valence bond theory which explains that structure as well as magnetic property of coordination compound. According to valence bond theory, metal ions or atoms under influence of ligands use (n−1)d,ns,np along with d orbital for hybridization. Thus yields a set of equivalent orbits that define geometry in terms of octahedral, tetrahedral and square planar. These hybridized orbits can also overlap with the ligand orbital to donate electron pairs for the bonding purpose.
Complete step-by-step answer: As we know that molecular formula of N(SiH3)3as well as it has pπ−dπ bonding present. From the molecular formula we get to the concept of back-bonding is followed here.
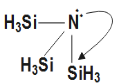
N(SiH3)3 is example where back-bonding is involved in above diagrams due to presence of empty 3d orbitals in Si atom lone pair N-atom get coordinate which is in empty 3d orbital of Si leading with formation pπ−dπ co-ordinate bond which leads to change from sp3 N-atom to sp2 N-atom. Thus it forms a trigonal planar molecule, also from the diagram below it is clear that the trigonal planar geometry is with all resonating structures.
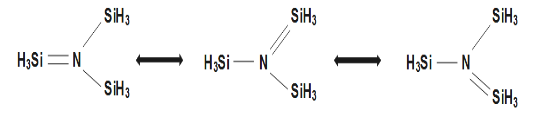
Therefore, option C is correct option i.e. Shape of (SiH3)3N with respect to N is Trigonal Planar.
Note: As we know, the structure of trimethylamine is pyramidal in shape due to the presence of bonding examples like trisilylamine. The trimethylamine molecular formula is given by (SiH3)3N where N undergoes a hybridization process as well as shape is pyramidal. Therefore, it's more basic than the former compound.
