Question
Question: Select the INCORRECT statement about product(s) of the reaction. $trans-hex-3-ene \xrightarrow{Br_2...
Select the INCORRECT statement about product(s) of the reaction.
trans−hex−3−eneCCl4Br2Product(s)
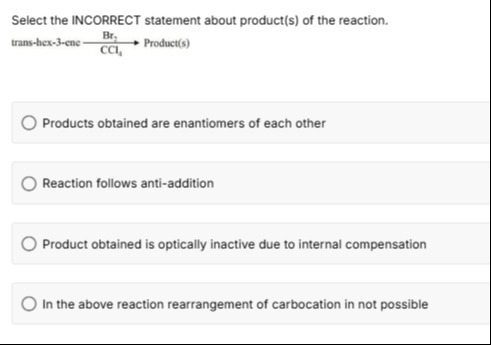
Products obtained are enantiomers of each other
Reaction follows anti-addition
Product obtained is optically inactive due to internal compensation
In the above reaction rearrangement of carbocation in not possible
Product obtained is optically inactive due to internal compensation
Solution
The reaction of trans-hex-3-ene with Br2/CCl4 follows anti-addition, forming a racemic mixture of (3R,4R) and (3S,4S)-3,4-dibromohexane. These are enantiomers. A racemic mixture is optically inactive due to external compensation (cancellation of optical rotation by equal amounts of enantiomers), not internal compensation (characteristic of meso compounds). The reaction proceeds via a cyclic bromonium ion, not a free carbocation, so rearrangements are not possible. Thus, the statement claiming optical inactivity due to internal compensation is incorrect.
