Question
Question: Select the Incorrect Reaction (s). ...
Select the Incorrect Reaction (s).
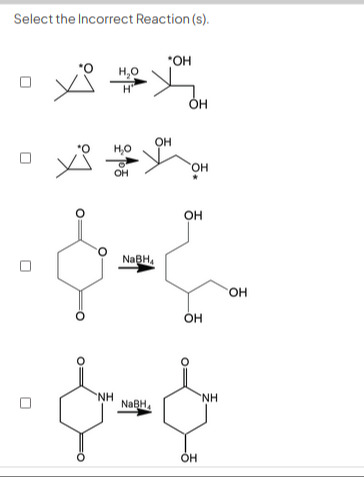
Reaction 1: Acid-catalyzed ring opening of 2,2-dimethyloxirane.
Reaction 2: Base-catalyzed ring opening of 2,2-dimethyloxirane.
Reaction 3: Reduction of a cyclic anhydride with NaBH4.
Reaction 4: Reduction of a cyclic imide with NaBH4.
Reaction 3: Reduction of a cyclic anhydride with NaBH4.
Solution
The question asks to identify the incorrect reaction(s). Let's analyze each reaction.
Reaction 1: Acid-catalyzed ring opening of 2,2-dimethyloxirane. In acid-catalyzed opening of unsymmetrical epoxides, the nucleophile attacks the more substituted carbon due to greater stability of the transition state which has partial positive character on the more substituted carbon. The nucleophile is water. The product shown is correct.
Reaction 2: Base-catalyzed ring opening of 2,2-dimethyloxirane. In base-catalyzed opening of unsymmetrical epoxides, the nucleophile attacks the less hindered carbon (less substituted carbon) via an SN2 mechanism. The nucleophile is hydroxide ion (OH−), which comes from water. The product shown is correct.
Reaction 3: Reduction of a cyclic anhydride with NaBH4. The reactant is glutaric anhydride. NaBH4 is a reducing agent that reduces aldehydes and ketones to alcohols. It does not typically reduce esters or carboxylic acids. Anhydrides are more reactive than esters and carboxylic acids towards nucleophilic attack. NaBH4 in protic solvents can reduce anhydrides. The product shown is 1,3,5-pentanetriol. Starting from glutaric anhydride, which has 5 carbons, it is possible to get a 5-carbon product. However, the positions of the hydroxyl groups are unusual for a reduction of glutaric anhydride. Reduction of glutaric anhydride with LiAlH4 also yields 1,5-pentanediol. Reduction of glutaric anhydride with NaBH4 yields 5-hydroxyvaleric acid. Therefore, the product shown in reaction 3 is incorrect.
Reaction 4: Reduction of a cyclic imide with NaBH4. The reactant is glutarimide. Glutarimide is a cyclic imide. Imides contain two carbonyl groups attached to a nitrogen atom. NaBH4 can reduce carbonyl groups. However, imides are generally less reactive towards reduction by NaBH4 compared to aldehydes and ketones. The product shown is 5-hydroxy-2-piperidinone. This is consistent with the reduction of one of the carbonyl groups of glutarimide by NaBH4. Therefore, reaction 4 is correct.
Based on the analysis, reaction 3 is incorrect. Reactions 1, 2, and 4 appear to be correct representations of the reactions. The question asks to select the incorrect reaction(s).
