Question
Question: Select the final product of the following rearrangement reaction of bicyclic diol from the given com...
Select the final product of the following rearrangement reaction of bicyclic diol from the given compounds.
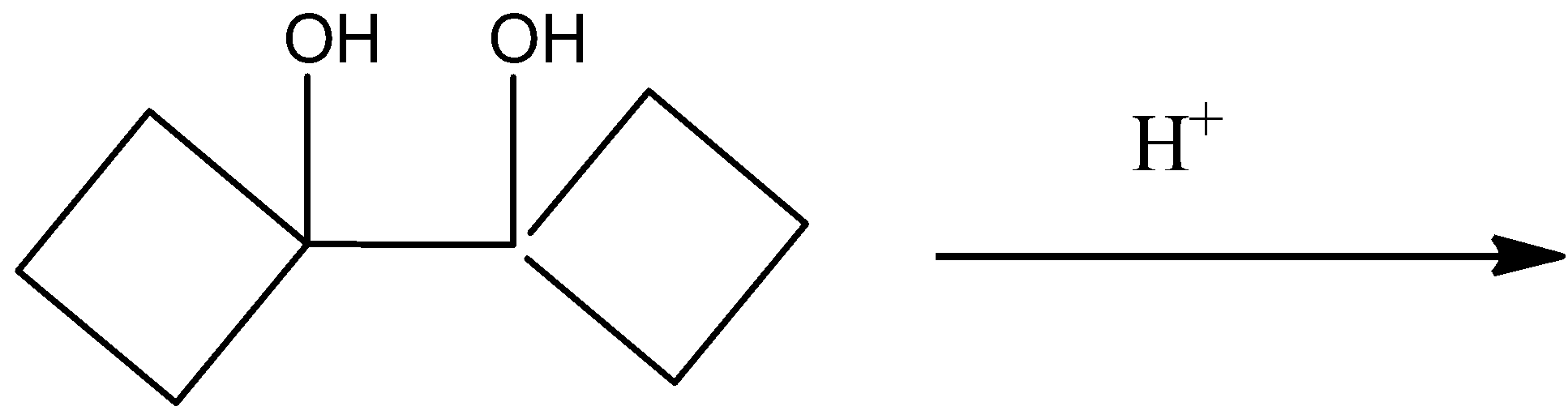
A)
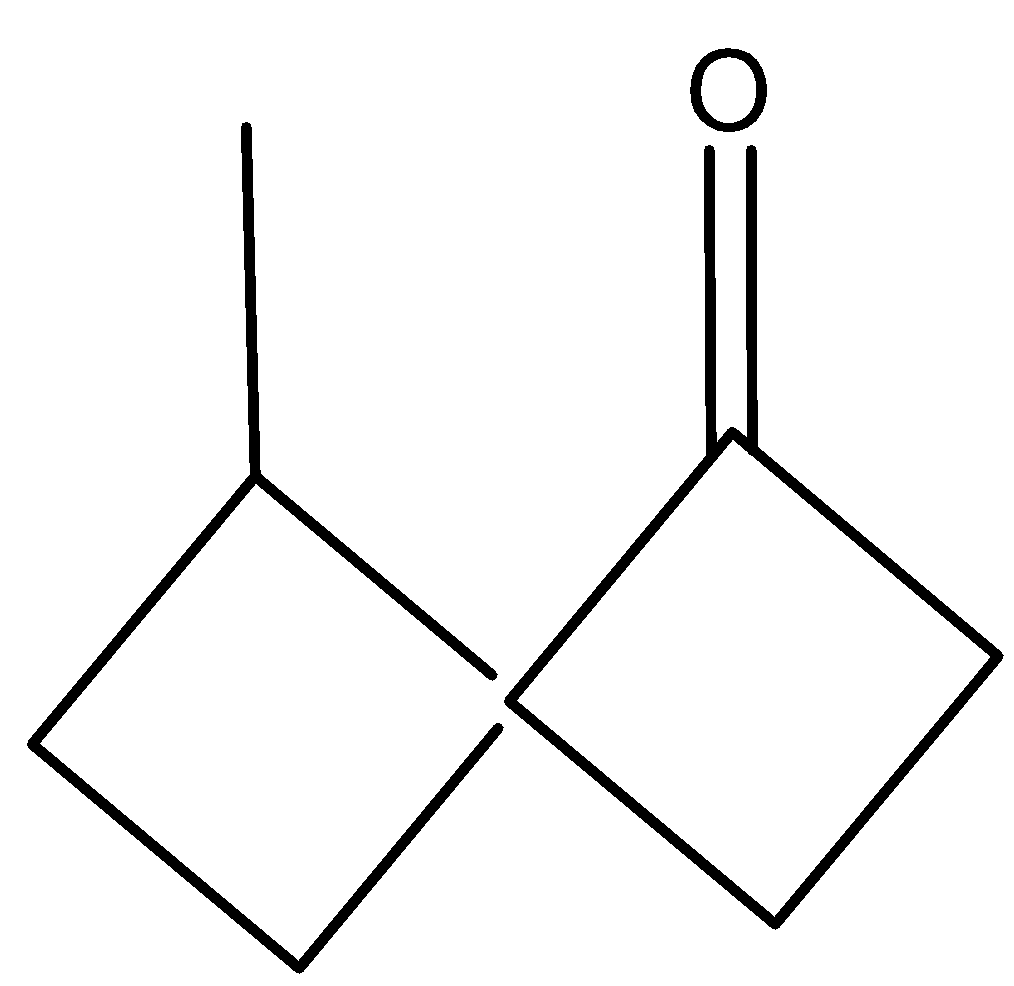
B)
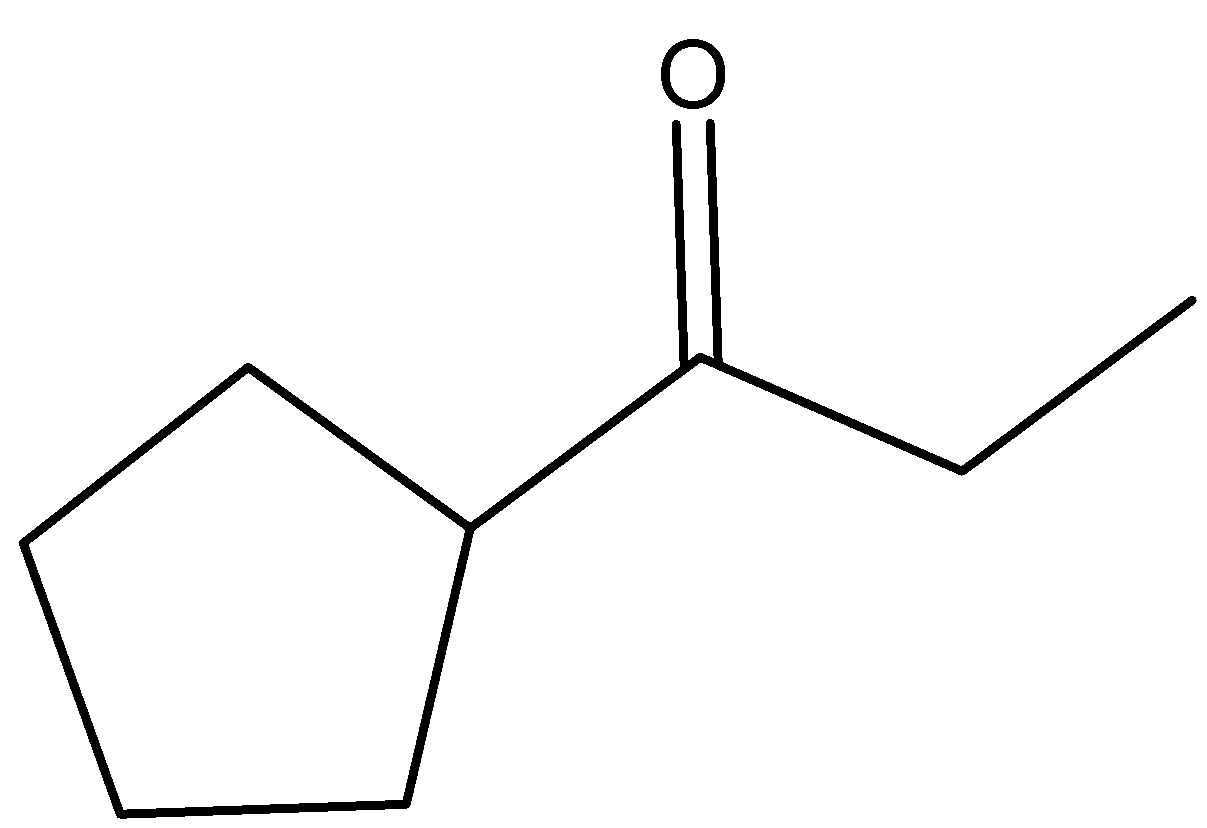
C)
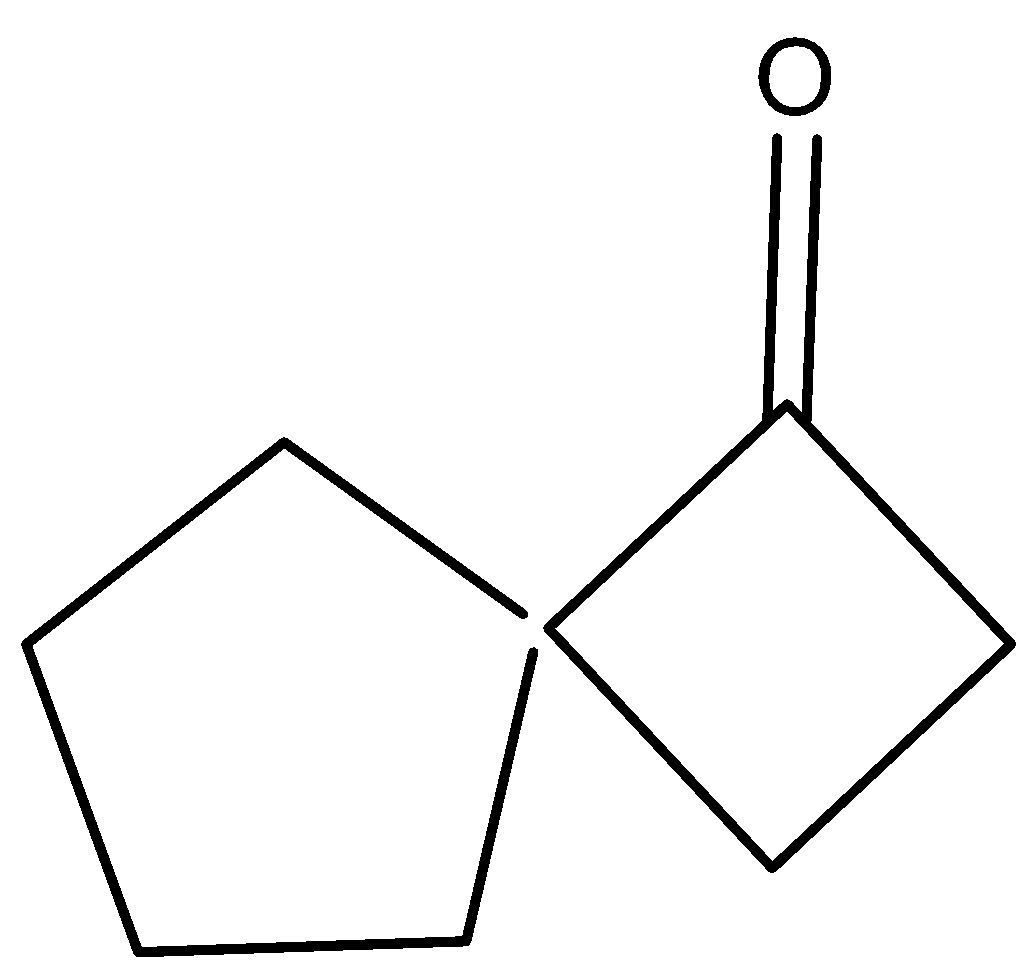
D)
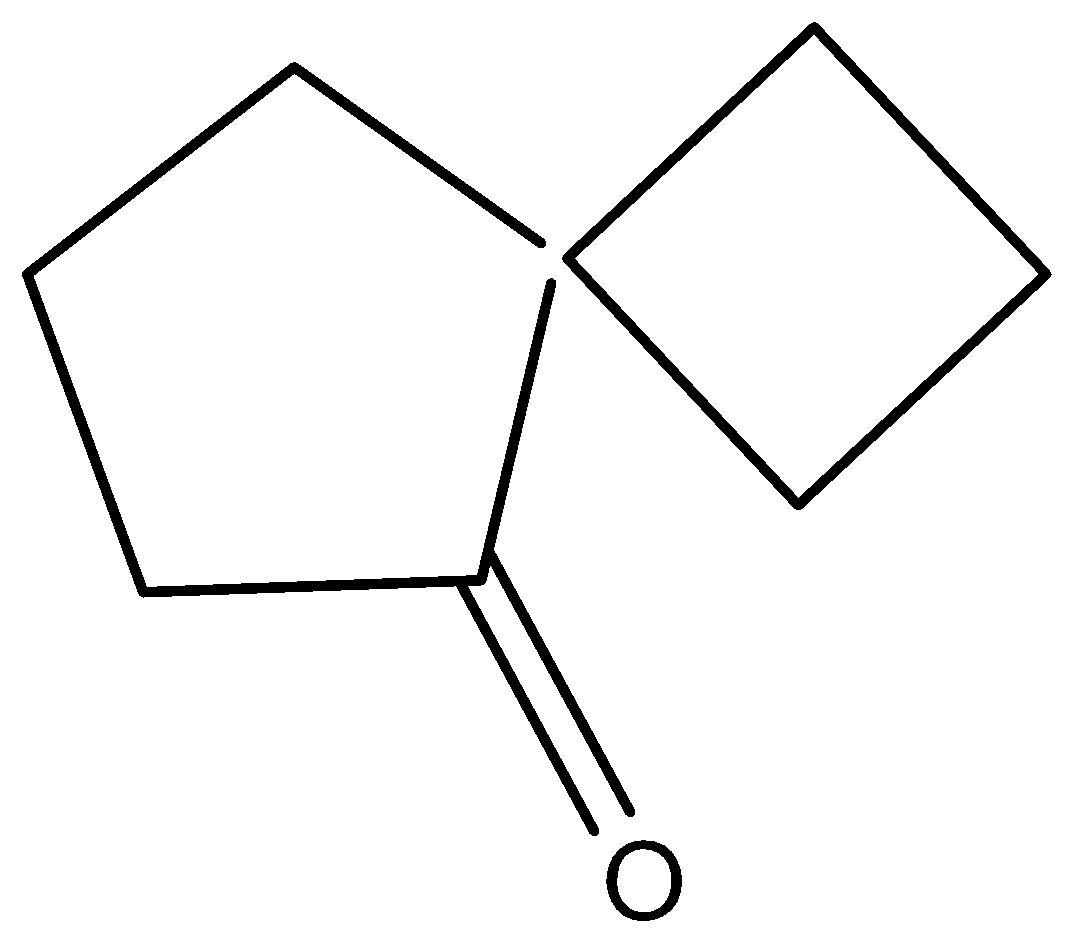
Solution
We know that the acid catalyzed rearrangement of vicinal diol to ketones or aldehydes with an elimination of water is understood as pinacol pinacolone rearrangement.
Complete step by step answer:
We know that the Pinacol Pinacolone rearrangement may be a vital process in chemistry for the conversion of 1,2 diols into carbonyl compounds containing a carbon oxygen covalent bond. This is often done via a 1,2 -migration which takes place under acyl conditions. Pinacol may be a compound which has two hydroxyl groups, each attached to a vicinal atom. It’s a solid compound which is white.
The Pinacol Pinacolone rearrangement mechanism takes place using four steps. Now we discuss the steps involved in pinacol-pinacolone.
1.Since the reaction is administered in an acidic medium, the hydroxide group of the pinacol is protonated by the acid.
2.Water is now far away from the compound, leaving a carbocation. This carbocation is tertiary and thus stable.
3.The methyl shifts to the charged carbon during a rearrangement of the compound.
4.The oxygen atom which is doubly bonded to the carbon is now deprotonated, giving rise to the specified pinacolone.
The completed reaction is given by,
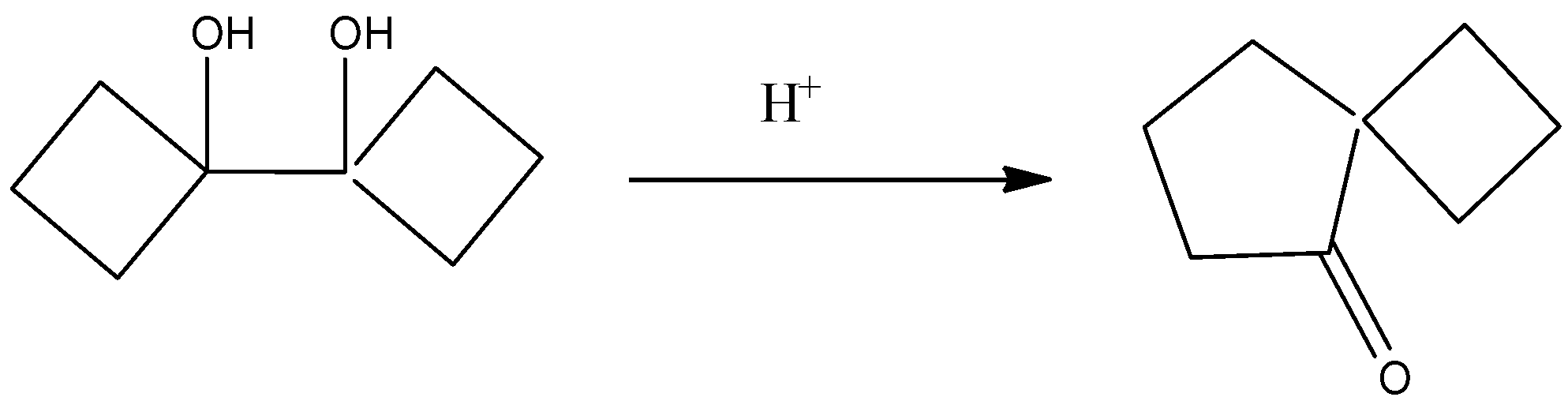
So, the correct answer is Option D .
Note:
Now we discuss about the uses of the pinacolone formed from the pinacol pinacolone rearrangement are:
Pinacolone is employed in Pesticides, Fungicides, and Herbicides.
Pinacolone is employed to organize the cyan guanidine drug – pinacidil.
Pinacolone is employed to supply triadimefon which is employed to regulate fungal diseases in agriculture.
