Question
Question: Select the correct statement(s) regarding buffer solution: $CH_3COOH(X \text{ molar}) + CH_3COONa(Y ...
Select the correct statement(s) regarding buffer solution: CH3COOH(X molar)+CH3COONa(Y molar)
At constant buffer concentration, buffer capacity increases to its maximum value if XY approaches 1.
At constant buffer concentration, variation of index of buffer with Y is parabolic in nature.
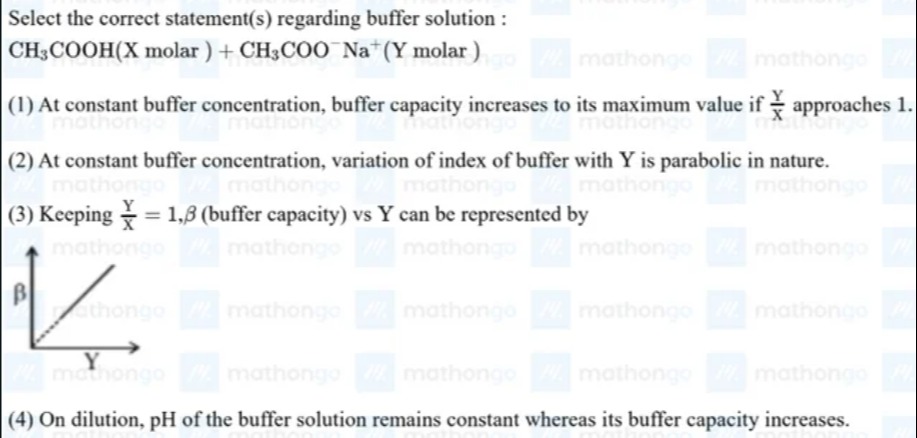
Keeping XY=1,β (buffer capacity) vs Y can be represented by
On dilution, pH of the buffer solution remains constant whereas its buffer capacity increases.
(1), (2), (3)
Solution
The buffer solution consists of a weak acid (CH3COOH, concentration X) and its conjugate base (CH3COO−, from CH3COONa, concentration Y).
Statement (1): At constant buffer concentration, buffer capacity increases to its maximum value if XY approaches 1.
The buffer capacity (β) for a weak acid-conjugate base buffer is given by β=2.303[Acid]+[Salt][Acid][Salt]. Let the total buffer concentration be C=[Acid]+[Salt]=X+Y. Then β=2.303X+YXY=2.303CXY. We want to maximize β for a constant C. We know that for a fixed sum X+Y=C, the product XY is maximum when X=Y. If X=Y, then XY=1. So, at constant buffer concentration, the buffer capacity is maximum when the ratio XY=1. As the ratio XY approaches 1, the buffer capacity increases towards its maximum value. This statement is correct.
Statement (2): At constant buffer concentration, variation of index of buffer with Y is parabolic in nature.
Let the constant buffer concentration be C=X+Y. So X=C−Y. The buffer capacity is β=2.303X+YXY=2.303C(C−Y)Y=C2.303(CY−Y2). This is a quadratic function of Y of the form β=aY2+bY where a=−C2.303 and b=2.303. The graph of a quadratic function is a parabola. Since the coefficient of Y2 is negative, the parabola opens downwards. The variable Y represents concentration, so Y≥0. Also, X=C−Y≥0, so Y≤C. Thus, the domain of Y is 0≤Y≤C. The variation of buffer capacity with Y is parabolic in nature over this range. This statement is correct.
Statement (3): Keeping XY=1, β (buffer capacity) vs Y can be represented by the given figure.
If XY=1, then Y=X. The buffer capacity is β=2.303X+YXY. Substituting X=Y, we get β=2.303Y+YY⋅Y=2.3032YY2=2.3032Y. So, β=(22.303)Y. This is a linear relationship between β and Y with a positive slope of 22.303 and a y-intercept of 0. The given figure shows a straight line passing through the origin with a positive slope, which represents a linear relationship between β and Y where β is directly proportional to Y. This is consistent with the derived equation. This statement is correct.
Statement (4): On dilution, pH of the buffer solution remains constant whereas its buffer capacity increases.
According to the Henderson-Hasselbalch equation, pH=pKa+log[Acid][Salt]=pKa+logXY. When a buffer solution is diluted by a factor D, the new concentrations are X′=X/D and Y′=Y/D. The new pH is pH′=pKa+logX′Y′=pKa+logX/DY/D=pKa+logXY=pH. So, the pH of the buffer solution remains constant on dilution (assuming concentrations are not extremely low). The original buffer capacity is β=2.303X+YXY. After dilution, the new buffer capacity is β′=2.303X′+Y′X′Y′=2.303(X/D)+(Y/D)(X/D)(Y/D)=2.303(X+Y)/DXY/D2=2.303D(X+Y)XY=D1(2.303X+YXY)=Dβ. Since dilution means D>1, the new buffer capacity β′ is less than the original buffer capacity β. So, on dilution, the buffer capacity decreases, not increases. This statement is incorrect.
Based on the analysis, statements (1), (2), and (3) are correct.
