Question
Question: Select the correct statement(s). A dipeptide of Glycine & Alanine, whose abbreviated name is GLY-AL...
Select the correct statement(s).
A dipeptide of Glycine & Alanine, whose abbreviated name is GLY-ALA
Compound
show Keto-enol tautomerism.
Phenol & benzoic acid can be distinguished by NaHCO3.
Order of basicity in aqueous medium MeNH2<Me2NH<Me3N
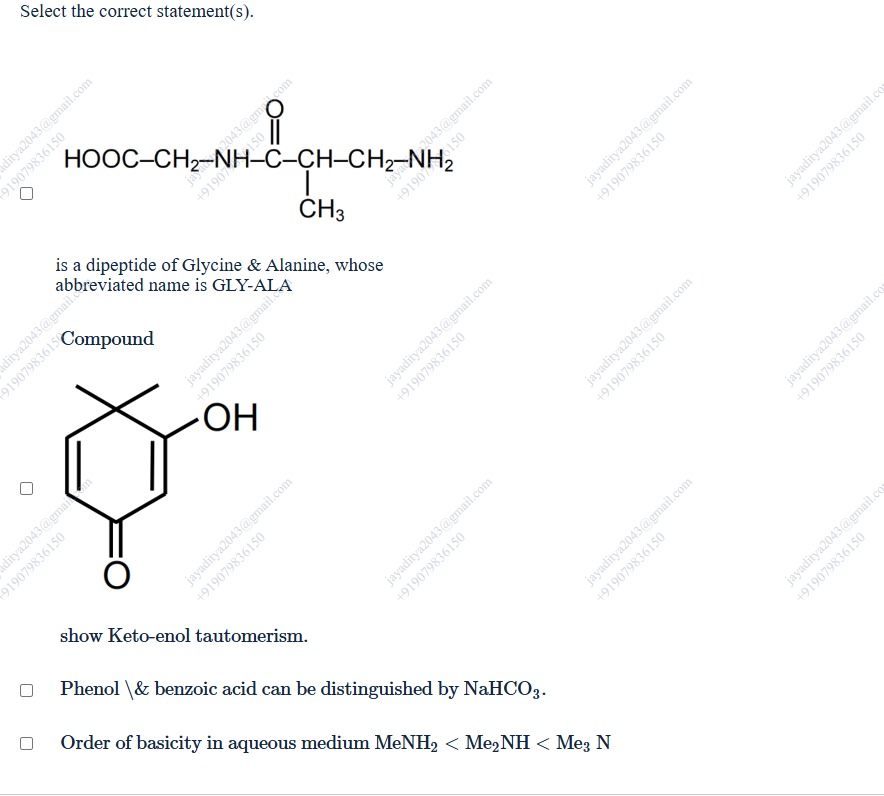
is a dipeptide of Glycine & Alanine, whose abbreviated name is GLY-ALA
Compound
show Keto-enol tautomerism.
Phenol & benzoic acid can be distinguished by NaHCO3.
Order of basicity in aqueous medium MeNH2<Me2NH<Me3N
Correct statements are options 2 and 3.
Solution
-
Dipeptide Structure:
H2N−CH2−CO−NH−CH(CH3)−COOH
A dipeptide of glycine and alanine in the conventional sequence isThe given structure is
HOOC−CH2−NH−C(O)−CH(CH3)−CH2−NH2which implies the free groups are at opposite ends (–COOH on one end and –NH₂ on the other) but the order is reversed compared to Gly–Ala. In fact, if we “read” it in the normal peptide direction it corresponds to Ala–Gly rather than Gly–Ala. Hence, the statement claiming it is Gly–Ala is incorrect.
-
Keto–Enol Tautomerism:
The second compound is described as a six‐membered ring with alternating single and double bonds and substituents: a methyl, a hydroxyl, and a carbonyl group. Such systems (for example, a cyclohexenone derivative) are known to exhibit keto–enol tautomerism. Therefore, this statement is correct. -
NaHCO₃ Test for Phenol vs Benzoic Acid:
Benzoic acid (pKₐ ≈ 4.2) reacts with sodium bicarbonate to form its salt due to its higher acidity, whereas phenol (pKₐ ≈ 10) does not react appreciably. Hence, this statement is correct. -
Basicity Order of Methylamines in Aqueous Medium:
Me2NH>MeNH2>Me3N
In water the basicity is affected by steric as well as solvation effects. Experimentally, the conjugate acid pKₐ values are such that dimethylamine is more basic than methylamine, but trimethylamine is less basic than dimethylamine. The order is generally:The given order MeNH2<Me2NH<Me3N is incorrect.
