Question
Question: Select the correct statements about diborane : (a) \( {B_2}{H_6} \) has three centred bound (...
Select the correct statements about diborane :
(a) B2H6 has three centred bound
(b) each boron atom lies in sp3 hybrid state
(c) Hb . . . . . B . . . . . . Hb bond angle is 122o
(d) All hydrogens in B2H6 lie the same.
Solution
Hint : The chemical compound diborane, sometimes known as diborane, has the formula B2H6 and is made up of boron and hydrogen. It's a colourless, pyrophoric gas with an odiferous sweet odour. Diborane is a common boron compound with a wide range of uses. Its electronic structure has gotten a lot of interest. It has a number of derivatives that are valuable reagents.
Complete Step By Step Answer:
The Diborane molecule is made up of four hydrogen atoms and two boron atoms that are all in the same plane. Two splitting hydrogen atoms are thought to exist between these planes. The boron atom possesses four hybrid orbitals and is known to be sp3 hybridised. Three of the four hybrid orbitals have one electron apiece, with the fourth orbital being an empty orbital. The two hybrid orbital electrons in each boron atom make two bonds with the 1s hydrogen atoms. The two boron atoms remaining with each unpaired electron orbital and empty orbital create two bridgings (B–H–B) bonds with the two 1s hydrogen atoms, which is also known as the banana bond.
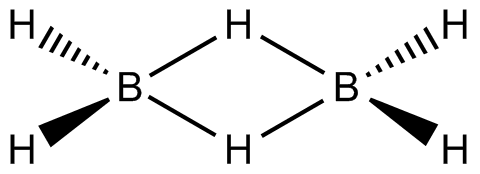
The links between boron and the terminal hydrogen atoms are described by molecular orbital theory as standard 2-center 2-electron covalent bonds. The connection between the boron atoms and the bridging hydrogen atoms, on the other hand, is not the same as in hydrocarbon molecules. Each boron bonds to the terminal hydrogen atoms with two electrons and has one valence electron left over for further bonding. Each of the bridging hydrogen atoms contributes one electron. Four electrons make two 3-center 2-electron bonds that hold the B2H2 ring together. A "banana bond" is a term used to describe this form of relationship.
Hence options A B C are correct.
Note :
Because the 12 valence electrons can only form 6 typical 2-centre 2-electron bonds, which are inadequate to bind all 8 atoms, diborane has traditionally been classified as electron-deficient. The more accurate description utilising 3-centre bonds, on the other hand, reveals that diborane is really electron-precise, with just enough valence electrons to cover the 6 bonding molecular orbitals.
