Question
Question: Select the correct statement(s). A. In diborane 12 valence \({e}^{-}\) are involved in bonding B...
Select the correct statement(s).
A. In diborane 12 valence e− are involved in bonding
B. In diborane, maximum six atoms, two boron and four terminal hydrogen, lie in the same plane.
C. Diborane has ethane-like structure.
D. In diborane, bridging bonds are stronger and longer than the terminal bonds.
Solution
Hint: Diborane is a chemical compound which consists of two elements, boron and hydrogen. The chemical formula of diborane is B2H6. It is used as a reducing agent and is used as a catalyst for hydrocarbon polymerization.
Complete step by step solution:
Diborane is a colorless, pyrophoric gas. It has a repulsively sweet odor. Let us now look at the options one by one.
(A). In diborane 12 valence electrons are involved in bonding: Let us first look at the structure of diborane.
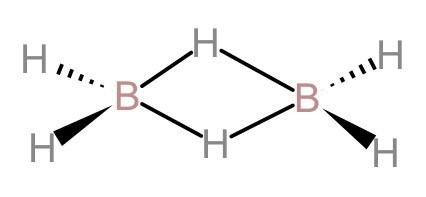
Now, by looking at the structure of diborane, we can see that there are 12 valence electrons that are involved in bonding. 8 valence electrons are used in the four non-bridging hydrogen bonds and four valence electrons are shared in forming 2-centered 2-electron bonds. Therefore, this statement is correct.
(B). In diborane, maximum six atoms, two boron and four terminal hydrogen, lie in the same plane: By looking at the structure of diborane, we can see that two boron atoms and the four terminal hydrogen atoms of the molecule are in the same plane. And the bridging hydrogen atoms lie above and below this plane. Therefore, this statement is also correct.
(C). Diborane has ethane-like structure: The structure of ethane has a single bond between the two carbon atoms but in diborane, there are bridged hydrogen-boron bonds. Therefore, it does not have an ethane-like structure. Hence, this is not correct.
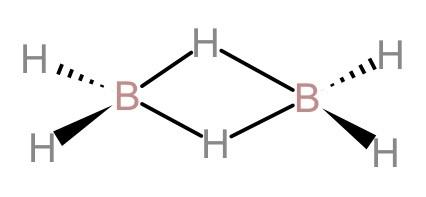
(D). In diborane, bridging bonds are stronger and longer than the terminal bonds: The bridging hydrogen bonds in diborane are stronger, which means that the bridging H-atoms cannot be easily replaced in a chemical reaction. The length of the bridging hydrogen-boron bonds is 1.33A0 which is longer than the length of the terminal hydrogen-boron length which is 1.19A0. And we know that, the longer the bond, the stronger the bond. Therefore, the bridging bonds are stronger and longer than the terminal bonds. Hence, this statement is also correct.
Therefore, the correct statements are (A), (B) and (D).
Note: Diborane is known to be an electron deficient compound. It is highly reactive and a versatile reagent. It is also used as a rocket propellant. The complete combustion of diborane is strongly exothermic.
