Question
Question: Select the correct statement for \({P_4}{O_{10}}\). (This question has multiple correct options) ...
Select the correct statement for P4O10.
(This question has multiple correct options)
A.It has four sp3 hybridised phosphorus atoms
B.It has high s% character in P-O bond than the P4O6
C.It has a cage like structure
D.It has pπ−dπ bonding
Solution
P4O10 is an oxide of phosphorus. Its chemical name is phosphorus pentoxide, because it is a dimer of P2O5 . In P4O10 , oxygen is in −2 oxidation state and phosphorus is in +5 oxidation state.
Complete step by step answer:
Structure of P4O10 is shown below.
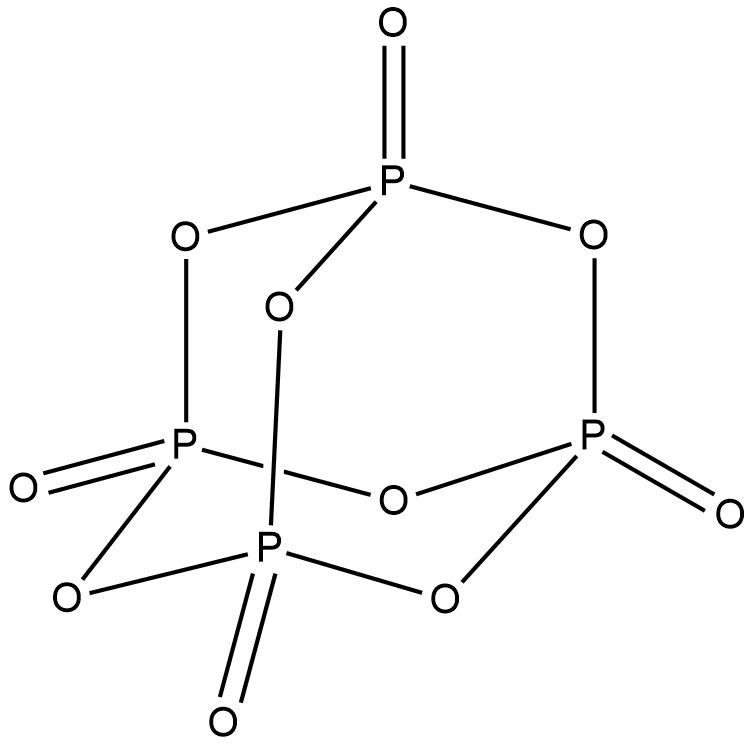
In P4O10 , all the phosphorus atoms are sp3 hybridised with a tetrahedral structure. sp3d hybridisation is preferred for phosphorus atom over sp3 hybridisation. But for P4O10 , sp3 hybridisation is more stable than sp3d. Hence P4O10has four sp3 hybridised phosphorus atoms. Option A is correct.
P4O6 is another oxide of phosphorus. Both P4O6 and P4O10 contains six P-O-P bond and 4 six membered ring. In P4O6 , the atomic orbitals containing lone pairs have more s-character and less p-character. Hence they have shorter bond length. But the bonding orbitals have more p-character and less s-character and hence a longer bond length. But in P4O10 the bonding orbitals have more s% character compared to that in P4O6 . The bonding orbitals means the P-O bond. Hence P4O6 has a higher s% character in P-O bond than the P4O6. Option B is also correct.
From the structure given above, we can see that P4O10 has a cage like structure. Hence option C is correct.
The terminal P-O bonds in P4O10 are formed by pπ−dπ bonding. p-orbitals of oxygen overlap with empty d-orbitals of phosphorus. The phosphorus atom donates its lone pair to this bonding forming a π - back bonding. Hence option D is correct.
Options A,B,C and D correct for P4O10 .
Note:
Phosphorus can show +3 and +5 oxidation states. Hence it forms two oxides P2O3 and P2O5 with oxidation states +3 and +5 respectively. But their monomeric form is not stable. This is the reason why they exist as dimer - P4O6 and P4O10.
