Question
Question: Select the correct statement about? A. All C atoms are \[s{p^2}\] -hybridised B. All C atoms are...
Select the correct statement about?
A. All C atoms are sp2 -hybridised
B. All C atoms are sp3- hybridised
C. All C atoms are sp - hybridised
D. None of the above
Solution
Benzene is present as a cyclic structure contains six carbons and six hydrogens. The alternated double bond ( conjugated system) is present in the benzene ring. The structure of benzene was first determined by Kekule. Kekule had a dream about a snake seizing its tail and thus, after that, he confirms that benzene must possess a cyclic structure.
Complete step by step answer:
Kekule proposed a benzene structure. The structure of benzene can be drawn as,
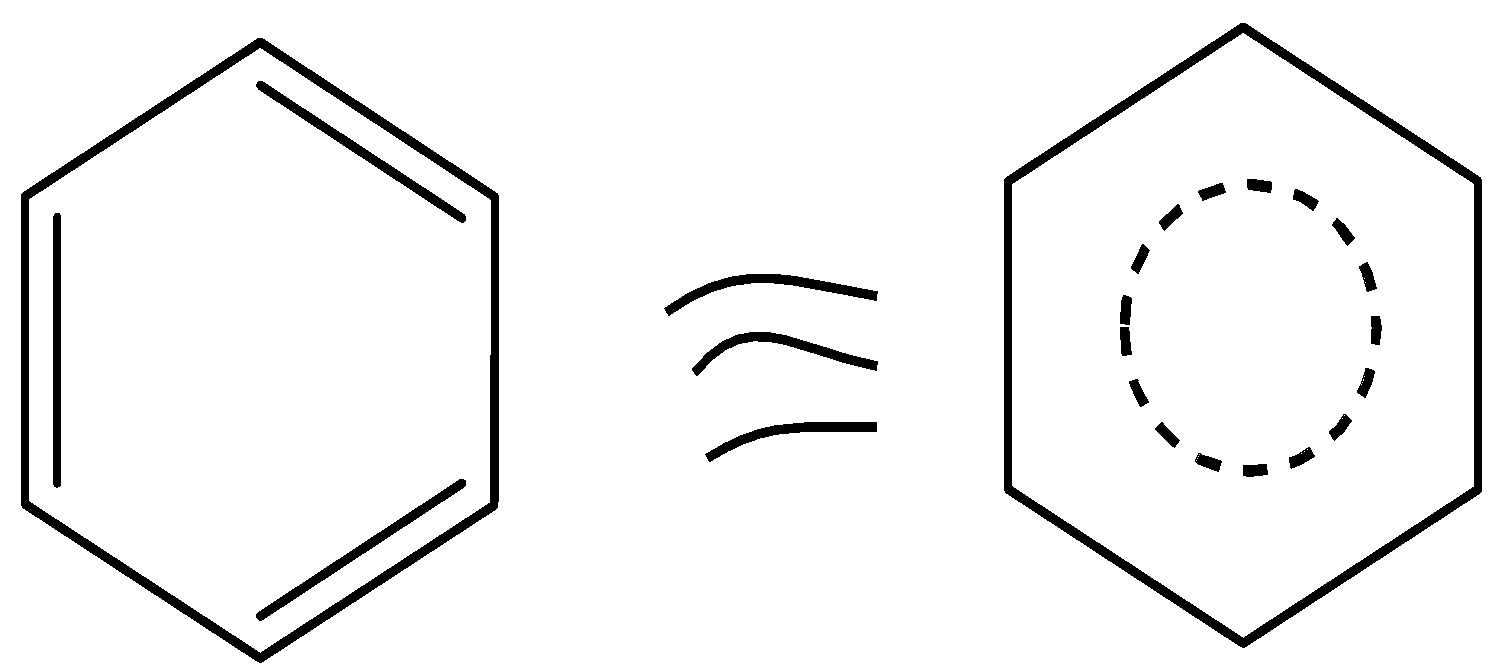
Thus, from that structure, it is clear that benzene contains six carbons and six hydrogens. Benzene alternately has three double bonds, thus it acts as a conjugate system. These characteristics define the aromaticity of the compound.
Benzene is involved in resonance and thus it exhibits maximum aromaticity when compared to other ring structures. The aromaticity of the compounds can be determined by Huckel’s (4n+2)πe− rule where n = 1,2,3 ….
In a benzene ring, it has three double bonds and each double bond contains two electrons. Benzene has six electrons. When n=1 in (4n+2)πe− rule, then we get the value as 6 similar to several double-bonded electrons in benzene. Thus, benzene is aromatic since it obeys Huckel's rule.
All carbon in the benzene ring is sp2 -hybridised since each carbon benzene has 3σ a bond and 1π. Sigma bond defines its hybridisation. If σ=3 , then the hybridization must be sp2.
Thus, All C atoms in the benzene ring are sp2 -hybridised.
Thus, the correct answer is option C.
Note: Sigma bond refers to a single bond and pi-bond refers to a double bond. If σ=2, then the hybridization must be sp. The best example of sp hybridisation in ethyne. If σ=4, then the hybridization must be sp3. The best example of sp3 hybridisation is methane.
