Question
Question: Select the correct option(s): :
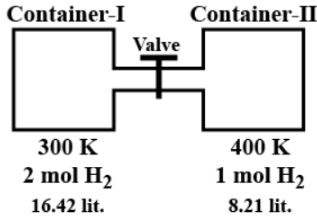
This question has multiple correct options
(A) Pressure in container-I is 3 atm before opening the valve.
(B) Pressure after opening the valve is 3.57 atm .
(C) Moles in each compartment are the same after opening the valve.
(D) Pressure in each compartment is the same after opening the valve.
Solution
Calculate the pressure in container-I and container-II using the ideal gas equation. Use the values given in the question. In equilibrium, the pressure and temperature will be the same in both the containers. Finally, calculate the pressure after the valve is opened and check the options that apply.
Complete answer:
We need to calculate the pressure in each container before opening the valve. To calculate pressure, we will use the ideal gas equation.
PV=nRT
Where, P→ pressure of gas
V→ volume of gas
n→ number of moles of gas
R→ universal gas constant =0.0821L atm mol−1K−1
T→ temperature of gas
To calculate pressure, we need to manipulate the above equation as follows:
P=VnRT
For container-I, let P1 the pressure, V1 be the volume, n1 be the no of moles and T1 be the temperature. Thus,
P1=V1n1RT1
P1=16.422×0.0821×300
On calculating further, we get,
P1=3 atm
This proves that option A is correct.
Similarly, for container-II:
P2=V2n2RT2
P2=8.211×0.0821×400
P2=4 atm
After the valve is opened, the gas inside the containers will try to reach equilibrium. Hence, pressure and temperature will be the same in both the containers.
Thus, option D is also correct.
Let the final temperature after equilibrium be Tf , then,
Tf=n1+n2n1T1+n2T2
On substituting values,
Tf=2+12×300+1×400
Tf=3600+400
On further solving,
Tf=31000=333.33K
Hence, the final temperature after the valve is opened is 333.33K .
Now, let the final pressure be Pf , then,
Pf=(V1+V2)(n1+n2)RTf
Pf=(16.42+8.21)(2+1)×0.0821×333.33
On simplifying,
Pf=24.633×0.0821×333.33
Pf=24.633×0.0821×333.33=3.33 atm
Hence, pressure after the valve is opened is 3.33 atm .
Thus, option B is incorrect.
Therefore, the correct options are A and D.
Note:
Option C is incorrect because, after equilibrium, the no of moles in each compartment will not be the same. While solving the equations, use the value of gas constant R as per the units used in question as the value changes when different units are used. For example, the value of R is 8.3145JK−1mol−1 and 1.9872calK−1mol−1 .
