Question
Question: Select the correct option(s). .
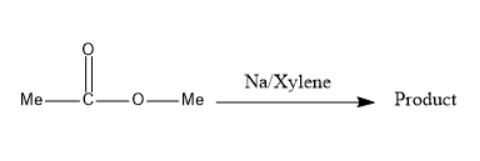
(A) Product is optically inactive
(B) Product is optically active
(C) In the given reaction free radical is formed as intermediate
(D) Product shows tautomerism
Solution
Esters are the chemical compounds consisting of RCOOR′ , when two molecules of esters are reacted in presence of sodium and xylene, the product formed consists of a ketone and alcohol functional group which can undergo tautomerism. The product formed via the formation of a free radical intermediate.
Complete answer:
Chemical compounds are classified into functional groups based on the groups present in it. Esters are the chemical compounds consisting of RCOOR′ , where R and R′ are sometimes same alkyl groups and sometimes different alkyl groups.
Two molecules of esters in presence of sodium and xylene form a product known as 3 -hydroxy butane 2 -one. Further this product undergoes proton shift which can be known as tautomerism and forms a product named as but−2−ene−2,3−diol .
The compound formed is a racemic mixture which means equimolar of enantiomers. Thus, the product is optically inactive. This reaction can be passed through the formation of a free radical intermediate.
Thus, option A, option C, and option D are the correct options.
Note:
Aprotic solvents like xylene, benzene, and toluene were not used in the reaction. It leads to the formation of other products rather than this acyloin condensation. When protic solvents like ethanol, water are used it proceeds a chemical reaction known as Bouveault-Blanc reduction in which an ester is reduced to an alcohol.
