Question
Question: Select the correct match A. \({\left[ {{\text{Mn}}{{\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \ri...
Select the correct match
A. [Mn(NH3)6]2 + inner orbital complex
B. [Co(H2O)6]3 + outer orbital complex
C. [Pt(Cl)4]2 + diamagnetic complex
D. K3[Cu(CN)4] paramagnetic complex
Solution
To determine the answer we should to determine the hybridization of complex. We will determine the valence electronic configuration of metal ions and we will check if the ligand is strong or weak. Based on the metal charge and ligand strength we will determine the vacant d orbital. If inner d-orbitals are involved in hybridization, the complex will be inner if not then outer. If the complex has unpaired electrons, the complex will be paramagnetic if not then the complex will be diamagnetic.
Complete solution:
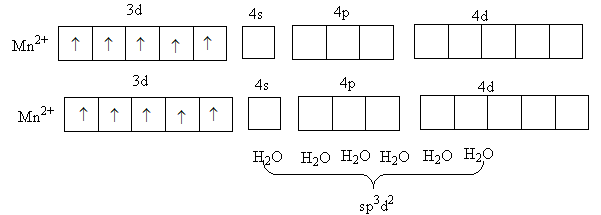
In the complex, [Mn(NH3)6]2 + metal manganese is in +2 oxidation state and ammonia is a weak field ligand. So, it will not cause the paring of d-electrons of metal.
The valence electronic configuration of Mn2 + is 3d5 .
So, each of the five d-orbital has one electrons so, no vacant 3 d orbital is available for hybridization so, Mn2 + will use its outer 4 d orbital for the hybridization and this the hybridization in [Mn(NH3)6]2 + will be sp3d2 so, [Mn(NH3)6]2 + is a outer orbital complex.
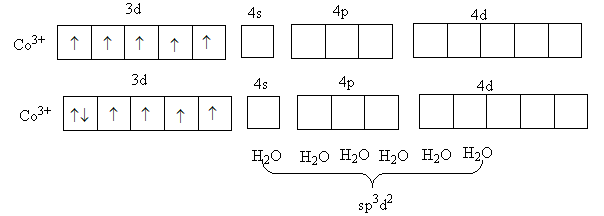
In the complex, [Co(H2O)6]3 + metal cobalt is in +3 oxidation state and water is a weak field ligand. So, it will not cause the paring of d-electrons of metal.
The valance electronic configuration of Co3 + is 3d6 .
So, four d-orbital has one electron and one d-orbital has two electrons so, no vacant 3 d orbital is available for hybridization so, Co3 + will use its outer 4 d orbital for the hybridization and this the hybridization in [Co(H2O)6]3 + will be sp3d2 so, [Co(H2O)6]3 + is a outer orbital complex.
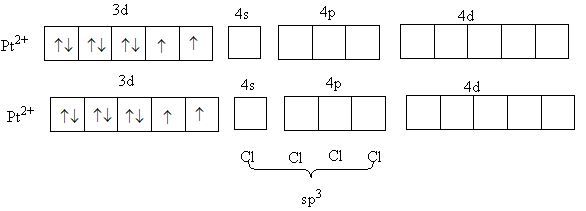
In the complex, [Pt(Cl)4]2 + metal platinum is in +2 oxidation state and chloro is a weak field ligand. So, it will not cause the paring of d-electrons of metal.
The valence electronic configuration of Pt2 + is 3d8 .
So, three d-orbital will have two electrons in each and two d-orbital will have one electron in each. So, two unpaired electrons are present in platinum (II) metal so the complex will be paramagnetic.
3
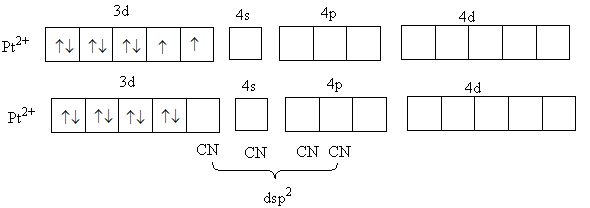
In the complex, K3[Cu(CN)4]metal copper is in + oxidation state and cyanide is a strong field ligand. So, it will cause the paring of d-electrons of metal.
The valence electronic configuration of Cu2 + is 3d8 .
So, four d-orbital will have two electrons in each. So, no unpaired electrons are present in copper (IiI) metal so the complex will be diamagnetic.
Therefore, option (B) [Co(H2O)6]3 + outer orbital complex, is correct.
Note: Inner orbital complexes are also known as low spin complexes and outer orbital complexes are also known as high spin complexes. In an octahedral complex, strong ligands cause the low spin or inner orbital complex. The weak ligand causes the high spin or outer orbital complex. In coordination number six, ligand strength affects the hybridization only whereas in coordination number four ligand strength affects the hybridization as well as the geometry. The strong ligand favours the square planar complex whereas the weak ligand favours the tetrahedral complex.
