Question
Question: Select the correct diagram for bonding molecular orbital which are formed by sideways overlapping. ...
Select the correct diagram for bonding molecular orbital which are formed by sideways overlapping.
A.
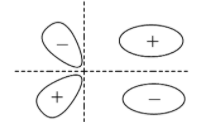
B.
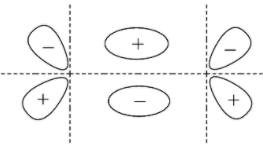
C.
D. None of these
This question has multiple correct options
Solution
Consider all the orbitals (s and p, since d and f are too complex to draw), and imagine their bonding and antibonding molecular orbitals. What does a sideways overlap in these orbitals show?
Complete answer:
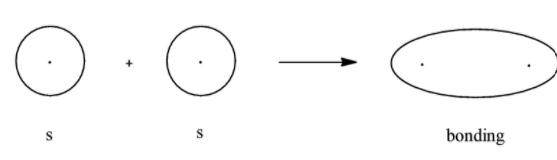
We know that s orbitals are spherical in shape. So, no matter how they overlap, it can be considered that they undergo sideways overlapping.
Recall the 2 lobes of the dumbbell shaped p- orbitals. In order for both the lobes to overlap, the orbitals have to overlap in a sideways fashion.
When the p orbitals overlap, if the orbitals are in phase then the resultant orbital is the bonding molecular orbital. If the 2 orbitals are out of phase then the orbitals formed will be the anti-bonding molecular orbitals. Let us first look at the bonding and antibonding molecular orbitals when 2 s orbitals combine.
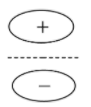
- Bonding molecular orbital for s
- Anti-bonding molecular orbital for s
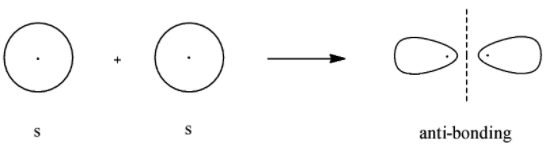
Here, we can see that the bonding molecular orbital mostly lies between the nuclei of the 2 atoms involved and the antibonding molecular orbitals lie in the space that is not between the 2 nuclei. Now we will look at the bonding and antibonding molecular orbitals of the p- orbital.
- Bonding molecular orbital for p
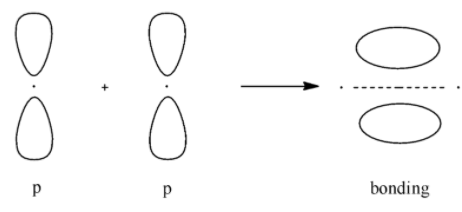
- Anti-bonding molecular orbital for p
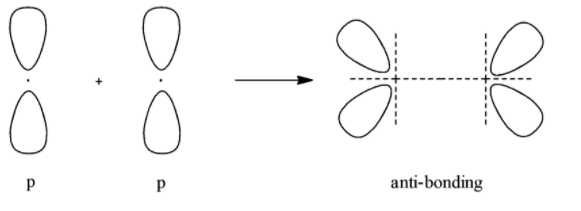
According to all these images, we can conclude that all the options given show the sideways overlap of the bonding orbitals of the p- orbital.
Hence the answers to this question are options ‘A’, ‘B’, and ‘C’ .
Note:
Remember that although the options show bonding molecular orbitals, the options ‘B’ and ‘C’ also show anti-bonding molecular orbitals. They are shown partially in option ‘B’ and completely in option ‘C’. So, if the question asks for a diagram that shows only the bonding molecular orbitals then mark only ‘A’ as the correct option.
