Question
Question: Select the basic strength order of following molecules ? ...
Select the basic strength order of following molecules ?
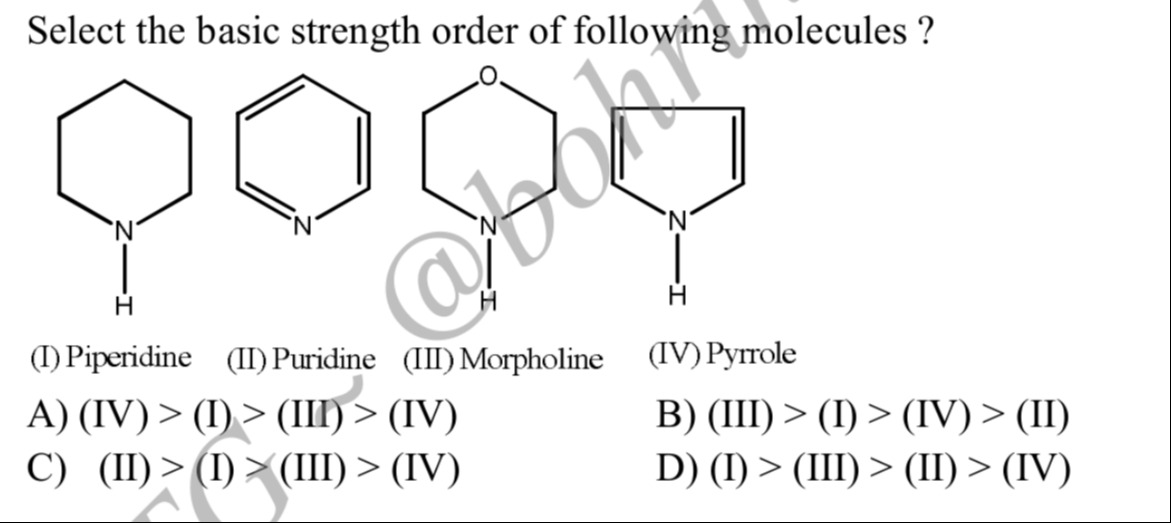
(I) > (III) > (II) > (IV)
(III) > (I) > (IV) > (II)
(II) > (I) > (III) > (IV)
(I) > (III) > (II) > (IV)
D
Solution
The basic strength of an amine depends on the availability of the lone pair of electrons on the nitrogen atom to accept a proton. This availability is influenced by factors such as the hybridization of the nitrogen atom, resonance effects, and inductive effects.
Let's analyze each compound:
-
Piperidine (I):
-
Structure: A saturated secondary amine.
-
Nitrogen Hybridization: sp3.
-
Lone Pair Availability: The lone pair is localized on the nitrogen atom and is readily available for protonation. There are no resonance effects that delocalize the lone pair.
-
-
Pyridine (II):
-
Structure: An aromatic heterocyclic amine.
-
Nitrogen Hybridization: sp2.
-
Lone Pair Availability: The lone pair of electrons resides in an sp2 orbital and is perpendicular to the π system, meaning it is not involved in the aromaticity. However, sp2 hybridized nitrogen is more electronegative than sp3 hybridized nitrogen (due to higher s-character), which means it holds its electrons more tightly, making the lone pair less available for protonation compared to saturated amines.
-
-
Morpholine (III):
-
Structure: A saturated secondary amine containing an oxygen atom in the ring.
-
Nitrogen Hybridization: sp3.
-
Lone Pair Availability: The lone pair is localized on the nitrogen. The adjacent oxygen atom is highly electronegative and exerts an electron-withdrawing inductive effect (-I effect) on the nitrogen. This reduces the electron density on the nitrogen, making its lone pair less available for protonation compared to piperidine.
-
-
Pyrrole (IV):
-
Structure: An aromatic heterocyclic amine.
-
Nitrogen Hybridization: sp2.
-
Lone Pair Availability: The lone pair of electrons on the nitrogen atom is part of the aromatic π system (contributes to the 6 π electrons that make the ring aromatic). If the nitrogen atom were to be protonated, it would disrupt the aromaticity, which is highly unfavorable energetically. Therefore, the lone pair is extremely unavailable for protonation, making pyrrole a very weak base.
-
Comparing Basic Strengths:
- Saturated vs. Aromatic Amines: Saturated amines (I and III) are generally more basic than aromatic amines (II and IV) because their nitrogen atoms are sp3 hybridized and their lone pairs are localized.
- Comparing I and III (Saturated Amines): Piperidine (I) has only carbon atoms in the ring, while morpholine (III) has an electronegative oxygen atom. The -I effect of oxygen in morpholine reduces the electron density on nitrogen, making it less basic than piperidine. So, (I) > (III).
- Comparing II and IV (Aromatic Amines): In pyridine (II), the lone pair is not part of the aromatic system. In pyrrole (IV), the lone pair is essential for aromaticity. Therefore, pyridine is significantly more basic than pyrrole. So, (II) > (IV).
- Comparing Saturated Amines with Pyridine: sp3 hybridized nitrogen is less electronegative than sp2 hybridized nitrogen. This means the lone pair is held less tightly in saturated amines (I and III) compared to pyridine (II). Thus, saturated amines are generally more basic than pyridine. So, (III) > (II).
Combining the comparisons, the order of basic strength is:
- Piperidine (I): Strongest base due to localized lone pair on sp3 nitrogen with no significant electron-withdrawing groups.
- Morpholine (III): Weaker than piperidine due to the electron-withdrawing effect of the oxygen atom, but still a relatively strong base due to sp3 nitrogen.
- Pyridine (II): Weaker than saturated amines due to sp2 nitrogen (higher electronegativity), but its lone pair is not involved in aromaticity.
- Pyrrole (IV): Weakest base because its lone pair is part of the aromatic system, and protonation would destroy aromaticity.
Therefore, the correct order of basic strength is: (I) > (III) > (II) > (IV).
