Question
Question: Select the appropriate relation with respect to acidity of X, Y, Z for the given compound, with incr...
Select the appropriate relation with respect to acidity of X, Y, Z for the given compound, with increasing order.
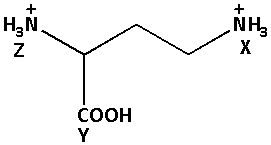
A) Z > X > Y
B) Y > Z > X
C) Z < X > Y
D) X > Y > Z
Solution
The species which donates hydrogen ions when dissolved in water is known as an acid. The acidic nature of a substance is directly proportional to the electronegativity of the groups attached. All the hydrogens are not acidic in nature. The hydrogen attached with electronegative atoms is acidic. The hydrogen atom attached to a carbon atom cannot be acidic unless some stabilizing factor balances the negative charge after hydrogen removal.
Complete solution:
We are given a structure as follows:
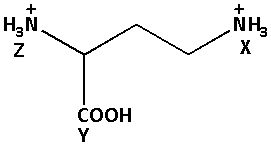
In the given structure, the carboxyl group (−COOH) is an acidic group and it can donate hydrogen ions more easily.
We know that the group which can donate hydrogen more easily is more acidic. Thus, the carboxyl group is the most acidic.
The displacement of sigma electrons due to the electron donating or electron accepting atoms or groups is known as inductive effect. The electron donating atoms or groups have positive inductive effect while the electron accepting groups or atoms have negative inductive effect.
The carboxyl group is an electron accepting or withdrawing group. Thus, it has a negative inductive effect.
The negative inductive effect decreases the electron density on the molecule. Thus, the electron donating ability of the molecule decreases i.e. the acidity increases.
The alkyl groups are electron donating groups. As the number of alkyl groups increase the electron density on the molecule increases.
As the distance of any group from the carboxyl group increases, the negative inductive effect decreases. As the negative inductive effect decreases the acidity decreases.
Thus, the NH3+ group at Z is more closer than the NH3+ group at Y. thus, the acidity of the NH3+ group at X is less.
Thus, the elation with respect to acidity of X, Y, Z for the given compound, with increasing order is:
Y > Z > X.
Thus, the correct option is (B) Y > Z > X.
Note: Remember that while deciding the acidic strength we have to check the strength of the inductive effect. As the distance increases, the negative inductive effect decreases and thus, the acidity also decreases. Remember that electron donating groups exert a positive inductive effect and electron withdrawing groups exert a negative inductive effect.
