Question
Question: Select the acid with the highest \({{K}_{a}}\)(i.e. lowest\(p{{K}_{a}}\)) A. 
A. 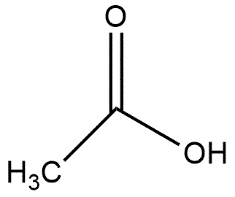
B. 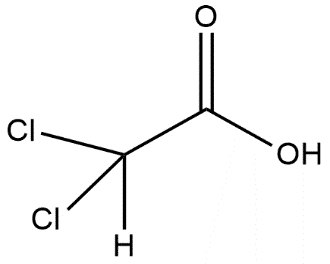
C. 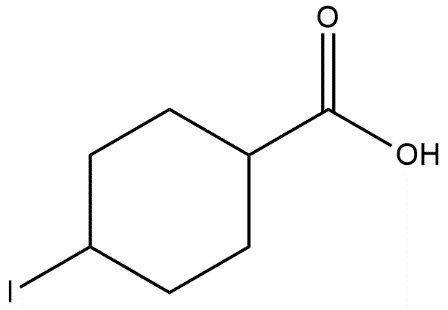
D. 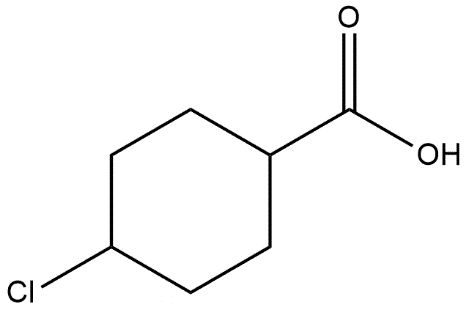
Solution
Ka,pKa,Kb,pKb values are helpful to predict whether a species will donate or accept protons at a specific pH value. These terms describe the degree of ionization of an acid or base. Ka,pKa relate to acids while Kb,pKbdeal with bases.
Complete answer:
When any electron releasing group or electron withdrawing group is attached with a chain of carbon atoms then partially positive or negative charge arises on the carbon chain atoms which causes permanent dipole in the molecule this type of effect is known as inductive effect. There are two types of inductive effect known as +I effect and –I effect.
-I effect occurs when an electronegative atom like halogen is attached to a chain of atoms resulting in unequal sharing of electrons which generates a positive charge. It causes a permanent dipole in the molecule wherein the electronegative atom holds a negative charge. On the other hand when an alkyl group is attached to a carbon chain then this is called +I effect.
Here in the option B carboxylic acid gives a proton and the anion which is left is stabilized by the –I effect and this shows that stronger will be the –I effect more will be stability and more will be the acidic strength. The presence of 2 chlorine atoms in option B shows the maximum –I effect and it has maximum acidic strength.
Thus option B is the correct answer.
Note:
Inductive Effect refers to the phenomenon by which a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds whereas the electromeric effect can only arise in pi bonds.
