Question
Question: Which of the following intermediate has 8 valence electrons around the carbon atom...
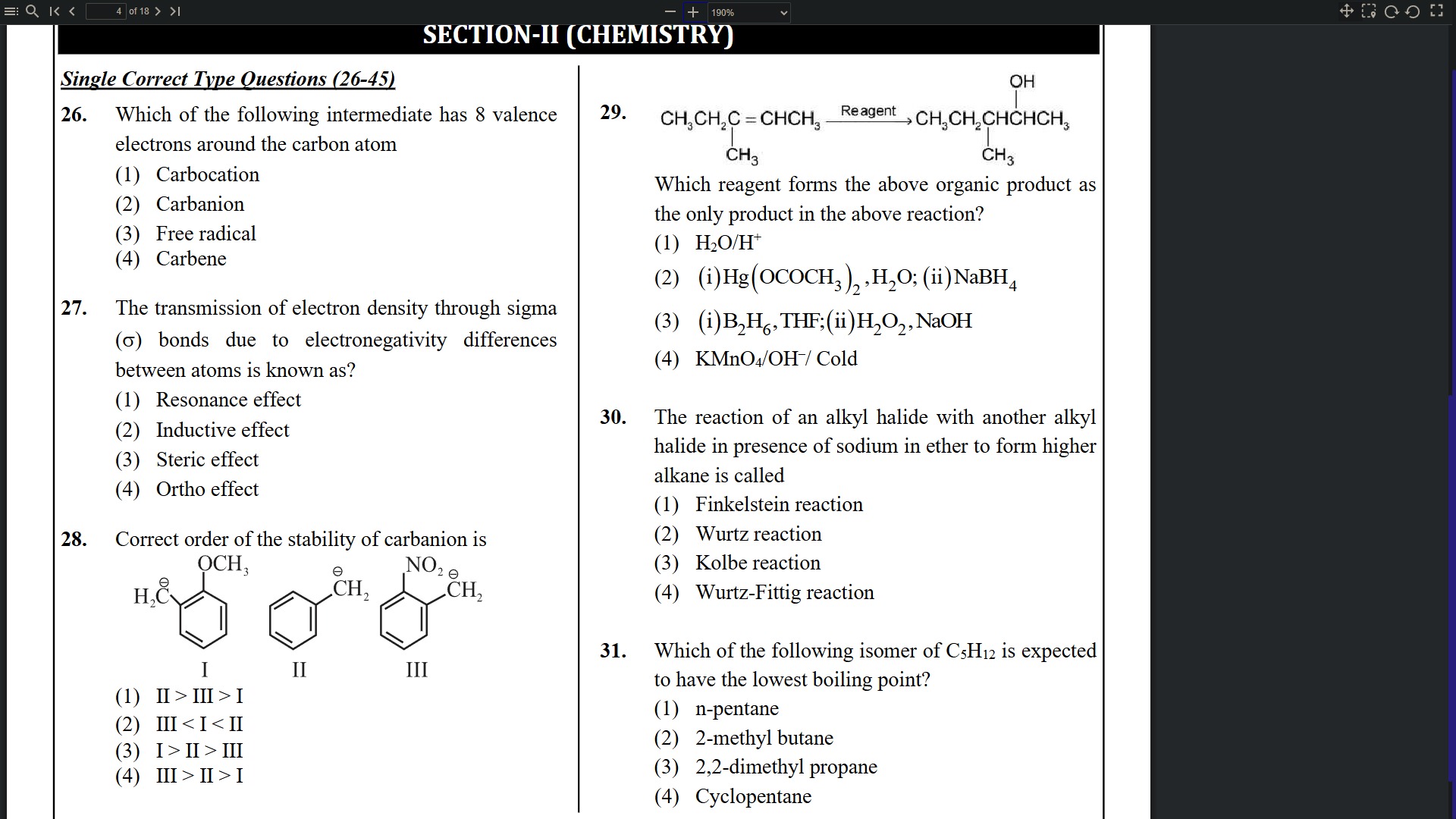
Which of the following intermediate has 8 valence electrons around the carbon atom
A
Carbocation
B
Carbanion
C
Free radical
D
Carbene
Answer
Carbanion
Explanation
Solution
A carbanion is an organic intermediate where carbon carries a negative charge and has a lone pair of electrons. It is sp3 hybridized and possesses a pyramidal geometry. The carbon atom forms three covalent bonds and has one lone pair, making a total of (3 * 2) + 2 = 8 valence electrons.
