Question
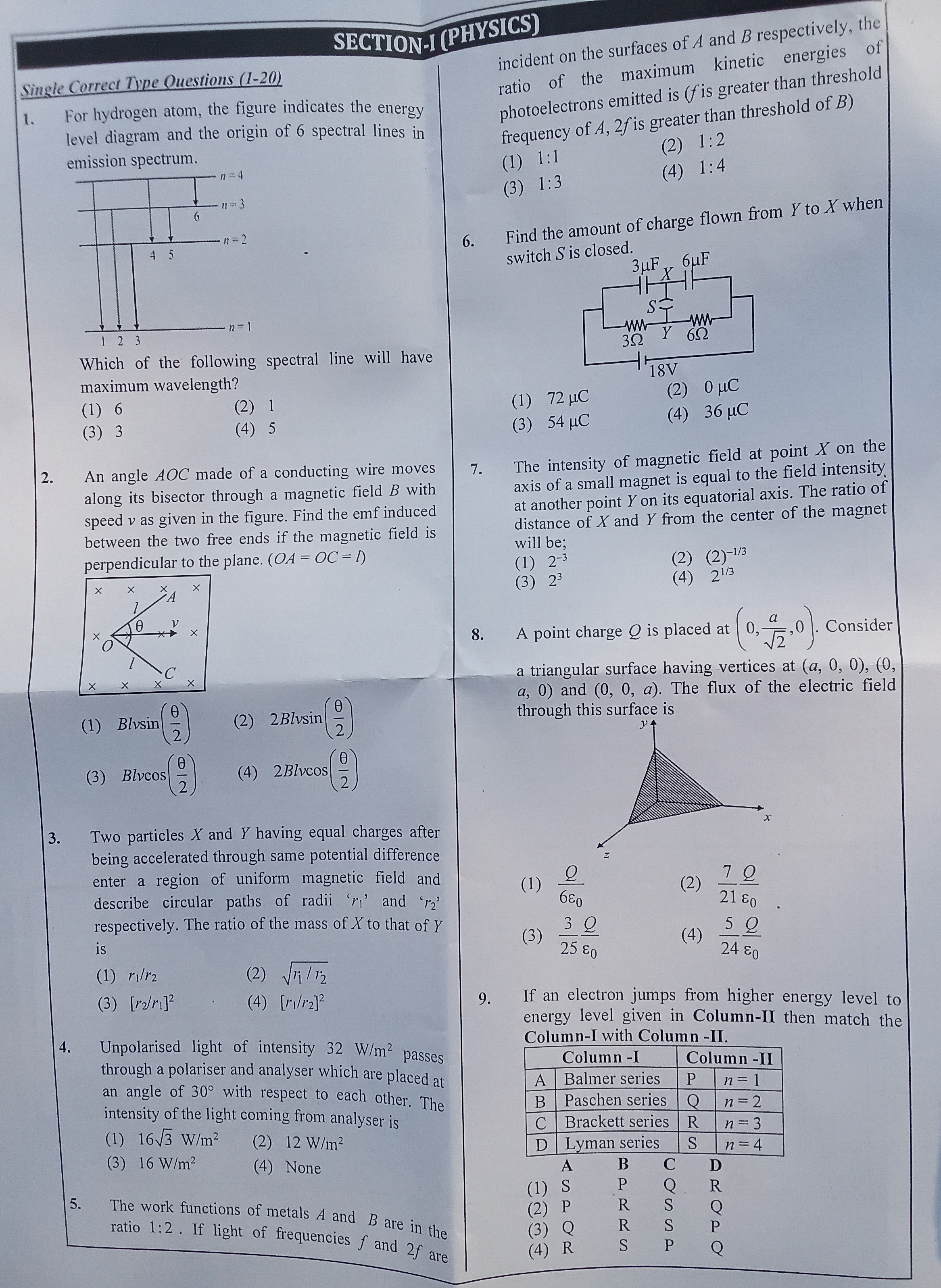
Question: For hydrogen atom, the figure indicates the energy level diagram and the origin of 6 spectral lines ...
For hydrogen atom, the figure indicates the energy level diagram and the origin of 6 spectral lines in emission spectrum.
Which of the following spectral line will have maximum wavelength?
A
6
A
3
B
1
D
5
Answer
6
Explanation
Solution
The energy of emitted photons is inversely proportional to their wavelength (ΔE=hc/λ). Maximum wavelength corresponds to minimum energy difference. The energy levels are En=−13.6/n2 eV. Line 6 is n=4→n=3. ΔE6=E4−E3=−13.6/16−(−13.6/9)=13.6(1/9−1/16)≈0.66 eV. Line 1 is n=4→n=2. ΔE1=E4−E2=−13.6/16−(−13.6/4)=13.6(1/4−1/16)≈2.55 eV. Line 3 is n=3→n=2. ΔE3=E3−E2=−13.6/9−(−13.6/4)=13.6(1/4−1/9)≈1.89 eV. Line 5 is n=2→n=1. ΔE5=E2−E1=−13.6/4−(−13.6/1)=13.6(1−1/4)≈10.2 eV. The minimum energy difference is for line 6.
