Question
Question: Secondary nitroalkanes on treatment with nitrous acid form: (A)- nitrolic acid (B)- carboxylic a...
Secondary nitroalkanes on treatment with nitrous acid form:
(A)- nitrolic acid
(B)- carboxylic acids
(C)- pseudonitroles
(D)- ketones
Solution
Nitrous acid is a weak and monoprotic acid known in solution, gas phase, and in the form of nitrite NO2− salts.
Complete step by step answer:
-If in a nitro hydrocarbon the hydrogen of an alkane is replaced by a nitro group it is known as a nitroalkane.
-Nitrous acid has the molecular formula HNO2 and is used in making diazonium salts from amines. The diazonium salts formed are used as reagents in the azo coupling reactions to give azo dyes.
-The reaction of nitrous acid with nitroalkanes helps us to distinguish between the primary, secondary and tertiary nitroalkanes. In this reaction, alpha-hydrogen is replaced by the nitroso group.
-If in an alkane the nitro group is attached to a terminal carbon, then it is known as a primary nitroalkane.
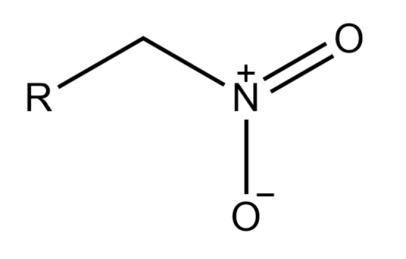
-If in an alkane the nitro group is attached to a saturated carbon atom which has two other carbons attached to it is known as Secondary nitroalkane.
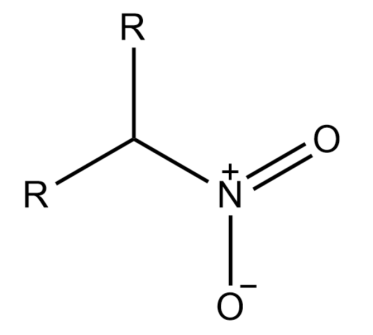
-Primary nitroalkanes can also react with nitrous acid forming a blue-colored nitroso-nitroalkanes (aci form) which on dissolution in sodium hydroxide gives a red solution.
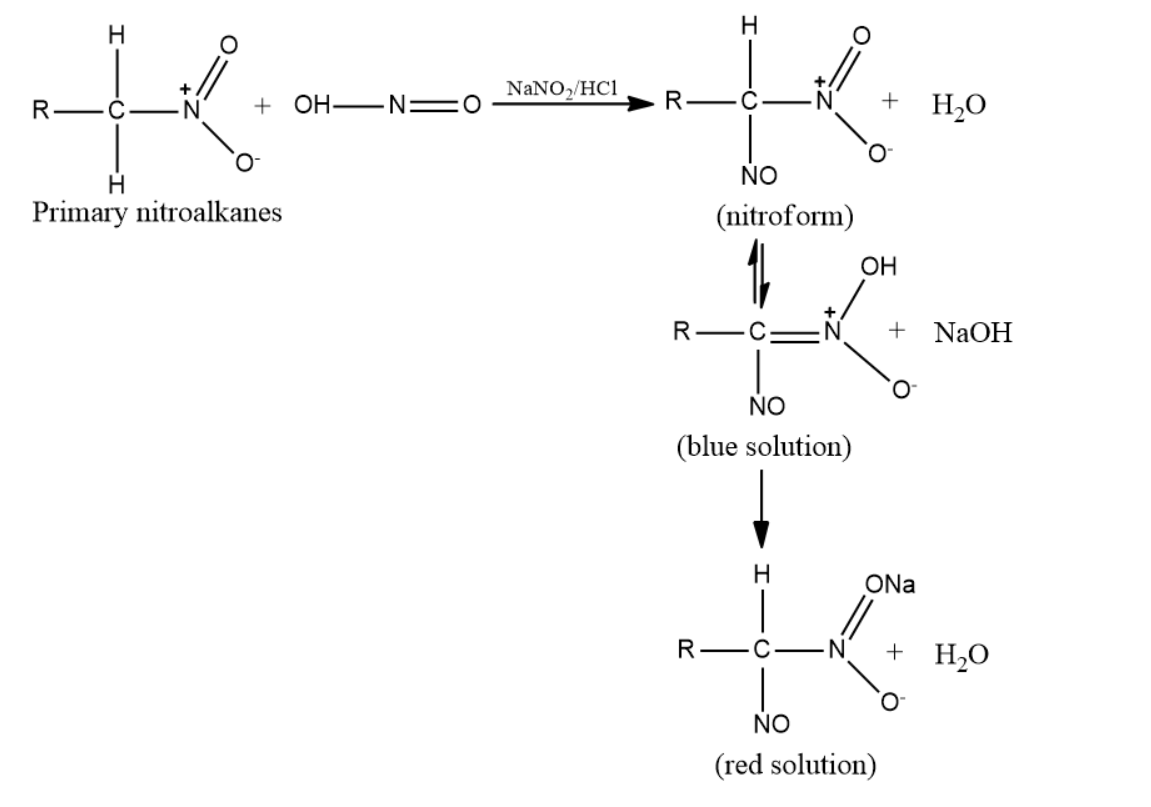
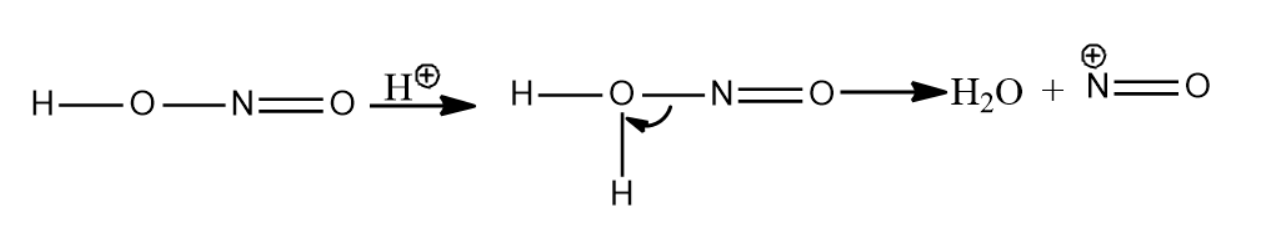
-Secondary nitroalkanes on reaction with nitrous acid give pseudonitroles. Nitrous acid is reduced to nitric acid.
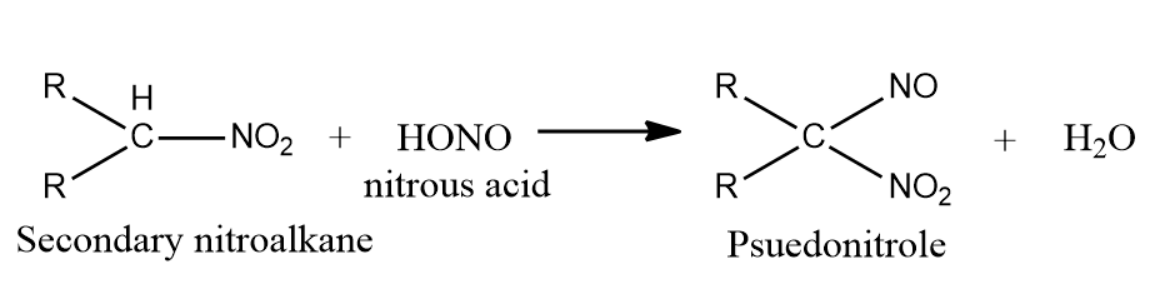
Pseudo nitrols are any class of compounds with general formula RR′C(NO)NO2 and have a pungent odour. They are actually colorless, but the solid dimmers formed in the reaction with nitroalkanes and nitrous acid when fused or dissolved polymerise into monomers of intense and characteristic blue color.So, the correct answer is “Option C”.
Note: You should not get confused with the nitrous acid as nitric acid. Nitrous acid has the molecular formula HNO2, whereas nitric acid has the molecular formula HNO3 .
