Question
Question: \(Sb{{F}_{5}}\) reacts with \(Xe{{F}_{4}}\) and \(Xe{{F}_{6}}\) to form ionic compounds \(\left[ XeF...
SbF5 reacts with XeF4 and XeF6 to form ionic compounds [XeF3+][SbF6−] and [XeF5+][SbF6−]. The geometry of XeF3+, [XeF5+] ion respectively is:
(A) square pyramidal, T-shaped
(B) bent T-shaped, square pyramidal
(C) see-saw, square pyramidal
(D) square pyramidal, see-saw
Solution
By obtaining the hybridisation of the central metal atom with respect to the ligands by considering the oxidation state and the configuration of the central atom. Then, from the lone pairs and the bond pairs, the geometry can be determined.
Complete step by step solution:
It is given that the SbF5 reacts with XeF4 and XeF6, acting as a Lewis acid as follows :
XeF4+SbF5→[XeF3]+[SbF6]−
XeF6+SbF5→[XeF5]+[SbF6]−
Now, in order to find the geometry of the cationic sphere, we will use the VSEPR theory as follows:
-In both the cations, xenon being least electronegative, is the central atom. Its electronic configuration at ground state is [Kr]4d105s25p6.
-Due to the presence of a (+1) charge on the sphere, that is, loss of an electron, the xenon loses one of its electrons from the 5p orbital. So, the configuration of Xe+ becomes [Kr]4d105s25p5.
-In case of XeF3+, in order to form three bonds with the fluorine atom. The xenon atom goes into an excited state and one of its 5p electrons jumps to 5d orbital. Now, these 5s, 5p and 5d-orbitals undergo hybridisation to form five sp3d hybrid orbitals, consisting of two lone pairs and three unpaired electrons.
-These unpaired electrons in the three hybrid orbitals overlap with an unpaired electron present in the three-fluorine p-orbital, one in each.
Thus, the hybridisation obtained in order to form XeF3+is sp3d hybridisation. So, the geometry will be trigonal bipyramidal, with one bond pair and two lone pairs in the equatorial position and the other two bond pairs in the axial position.
Due to the repulsion from the two lone pairs, we get a bent T-shaped molecule.
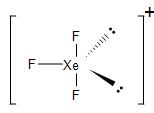
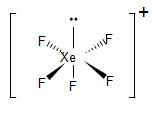
Similarly, in the case of XeF5+, in order to form five bond pairs with the fluorine atom. In the excited state, two p-electrons jump to the d-orbital. the 5s, 5p and 5d orbitals undergo hybridisation to form six sp3d2 hybrid orbitals. Thus, generating one lone pair and five unpaired electrons.
The five unpaired electrons overlap with the fluorine p-orbitals and form five bond pairs. Therefore, we get sp3d2 hybridisation and giving us an octahedral geometry. Due to the presence of the lone pair, its geometry gets distorted and we get a square pyramidal molecule.
Therefore, the shape of the XeF3+and XeF5+ entity is option (B)- bent T-shaped, square pyramidal.
Note: In order to obtain the hybridisation, the charge on the ion must be taken to obtain the oxidation state of the metal atom and then we would arrive at the appropriate hybridisation.
