Question
Question: Salicylaldehyde is obtained when phenol is heated with \(CHC{{l}_{3}}\) and aqueous \(NaOH\). This r...
Salicylaldehyde is obtained when phenol is heated with CHCl3 and aqueous NaOH. This reaction is known by which name?
(a)- Carbylamine reaction
(b)- Hoffmann’s reaction
(c)- Reimer-Tiemann reaction
(d)- Kolbe-Schmidt reaction
Solution
Salicylaldehyde is an organic compound in which the benzene ring has an alcohol group and at the ortho position an aldehyde functional group is present. The formation of salicylaldehyde from phenol was discovered by two scientists hence, its name contains both the names of scientists.
Complete Solution :
Salicylaldehyde is an organic compound in which the benzene ring has an alcohol group and at the ortho position, an aldehyde functional group is present. It is prepared from phenol with chloroform in the presence of sodium hydroxide.
- First, the phenol and chloroform are mixed in which there is an elimination of the HCl molecule. The OH group of the phenol converts into ONa and at the ortho position, there is an addition of CHCl2. Now in the presence of NaOH, both the chlorine atoms of CHCl2 will convert into hydroxyl ions. On heating this molecule there is the elimination of water molecules which will form an aldehyde functional group at the ortho position. Finally, this molecule is acidified which converts the ONa group back to OH group. The reaction is given below:
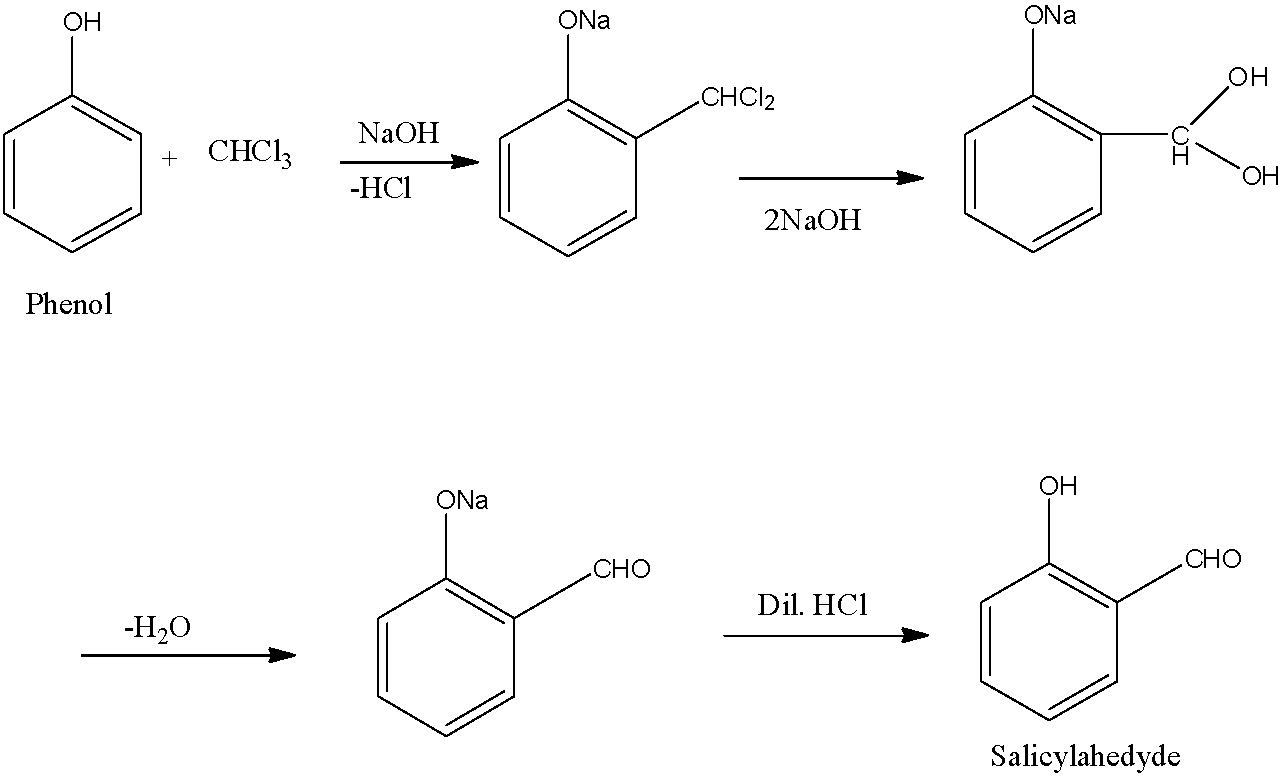
This reaction was discovered by two scientists Karl Reimer and Ferdinand Tiemann, so this reaction is named as Reimer-Tiemann reaction.
So, the correct answer is “Option C”.
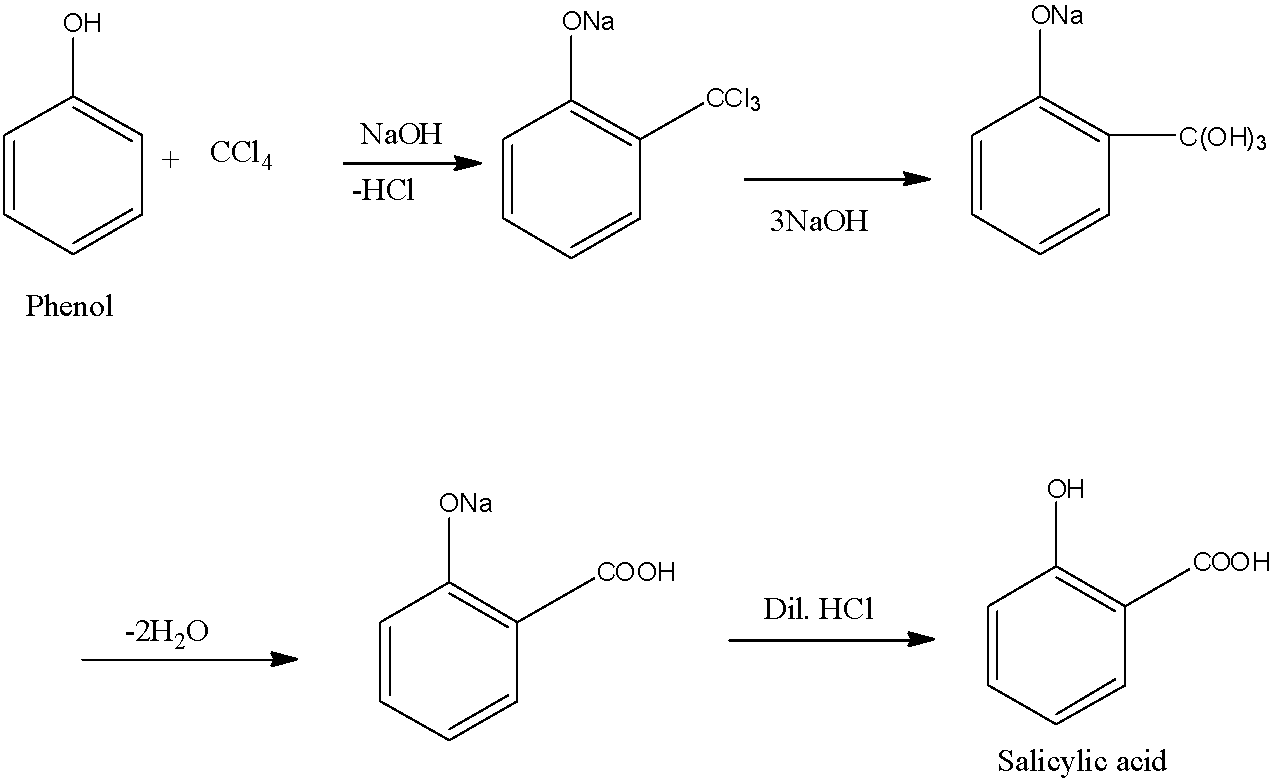
Note: Instead of chloroform, when we treat phenol with carbon tetrachloride, there is the formation of salicylic acid. The reaction process is the same and it is given below: