Question
Question: The standard EMF of the cell in which the reaction, $MnO_4^- + 5Fe^{2+} + 8H^+ \rightarrow Mn^{2+} +...
The standard EMF of the cell in which the reaction, MnO4−+5Fe2++8H+→Mn2++5Fe3++4H2O occurs is 0.59 V at 25°C. The log of equilibrium constant for the given reaction is approximately?
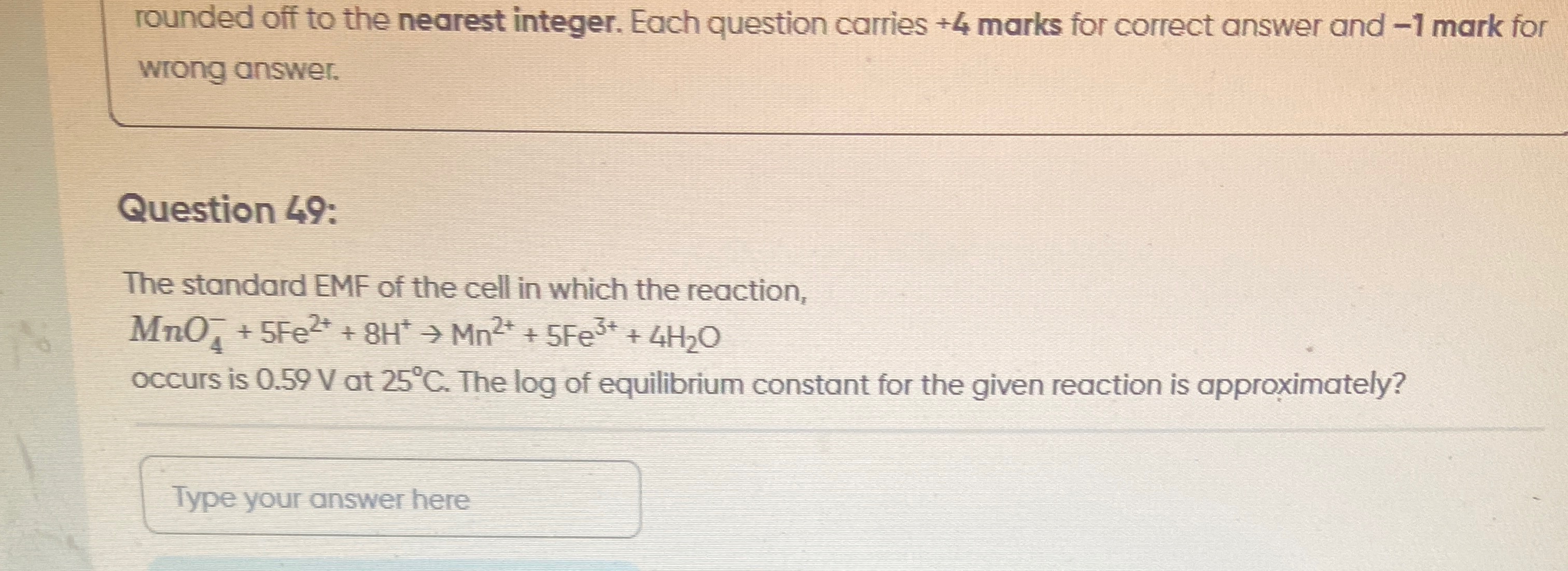
50
Solution
The relationship between the standard EMF (Ecell∘) and the equilibrium constant (K) of a reaction at 25°C is given by the Nernst equation at equilibrium:
Ecell∘=n0.0591logK
First, determine the number of electrons (n) involved in the given reaction: MnO4−+5Fe2++8H+→Mn2++5Fe3++4H2O
-
Oxidation state change for Mn: In MnO4−, Mn is in the +7 oxidation state. In Mn2+, Mn is in the +2 oxidation state. The change is from +7 to +2, which means a gain of 5 electrons (MnO4−+5e−→Mn2+). This is a reduction half-reaction.
-
Oxidation state change for Fe: In Fe2+, Fe is in the +2 oxidation state. In Fe3+, Fe is in the +3 oxidation state. The change is from +2 to +3, which means a loss of 1 electron (Fe2+→Fe3++e−). This is an oxidation half-reaction.
To balance the electrons lost and gained, 5 moles of Fe2+ must be oxidized for every 1 mole of MnO4− reduced. Thus, the total number of electrons (n) transferred in the balanced reaction is 5.
Given: Standard EMF (Ecell∘) = 0.59 V Number of electrons (n) = 5 Temperature = 25°C
Substitute the values into the Nernst equation: 0.59=50.0591logK
Now, solve for logK: logK=0.05910.59×5 logK=0.05912.95 logK≈49.91539
Rounding off to the nearest integer, logK is approximately 50.
Explanation of the solution:
The Nernst equation at equilibrium, Ecell∘=n0.0591logK, relates the standard cell potential (Ecell∘) to the equilibrium constant (K) and the number of electrons (n) transferred. For the given reaction, the oxidation state change of Mn from +7 to +2 indicates a gain of 5 electrons, and the oxidation state change of Fe from +2 to +3 indicates a loss of 1 electron. To balance the electron transfer, 5 Fe2+ ions are oxidized for every MnO4− ion reduced, so n=5. Substituting Ecell∘=0.59 V and n=5 into the equation yields logK=0.05910.59×5≈49.915, which rounds to 50.
