Question
Question: Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane ...
Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane is:
1. 360∘
2. 180∘
3. 120∘
4. 240∘
Solution
As we know that different conformations are the arrangement of two atoms in a molecule that are found to differ by rotation. And the study of energy between different conformations is called conformational analysis.
Complete Solution :
- By rotating the back carbon C2−C3 about axis by an angle of 180∘, the resulting conformation is called as Anti conformation or staggered conformation.
- We can see from the conformation drawn below:
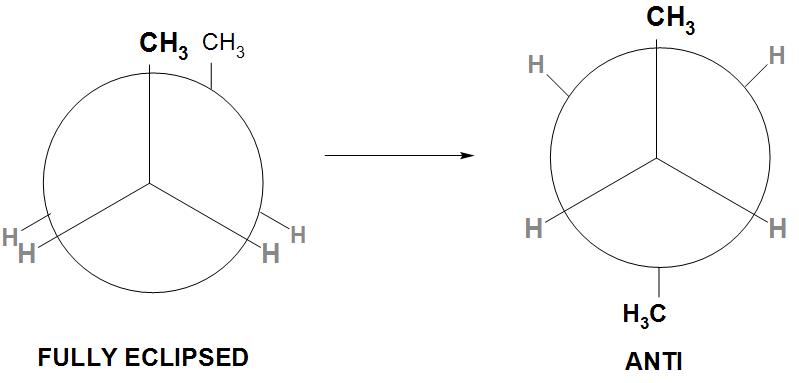
- We can see from the above staggered conformation that all the groups on C2 and C3 carbons are maximum apart. Therefore, it is found that the torsional and the steric interactions are minimum. ( the bulkier twoCH3 groups are at 180∘).
- Hence, it is found that this is the most stable and the most preferred conformation of n-butane. Therefore, its potential energy is considered as zero and the energies of all other conformation is determined relative to this.
- Hence, we can say that the correct option is (1), that is the Rotational angle required to get maximum stable conformer from minimum stable conformer in n-butane is 180∘
So, the correct answer is “Option 2”.
Note: - We should note the main difference in between eclipsed and staggered conformation. In eclipsed conformation, the carbons are aligned in such a way that the hydrogens are lined up with each other.
- In staggered conformation atoms are found to be equally spaced from each other.
