Question
Question: Roasting of sulphides gives the gas X as a product. This is a colorless gas with a cooking smell of ...
Roasting of sulphides gives the gas X as a product. This is a colorless gas with a cooking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic and acts as a reducing agent and its acid has never been isolated. The gas X is:
A.H2S
B.SO2
C.CO2
D.SO3
Solution
. This is a colourless gas with a choking smell of burnt sulphur and causes great damage to the respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated.
Complete step by step answer:
Roasting of sulphides gives us the gas SO2 as a byproduct. This is a colorless gas that has a cooking smell of burnt sulphur and causes great damage to respiratory organs as a result of the acid rain. Its aqueous solution is acidic and does act like a reducing agent and its acid has never been isolated.
For example sulphur dioxide gas is obtained by roasting zinc blende or iron pyrites.
2ZnS+3O2 →2ZnO+2SO2
4FeS2 +11O2 →2Fe2O3 +8SO2
Sulphur dioxide dissolves in water forming sulphurous acid. hence, it is known as sulphurous anhydride.
H2O+SO2=H2SO3
It reduces chlorine to HCl.
SO2+Cl2 +2H2O→H2SO4 +2HCl
Therefore it is SO2 gas.
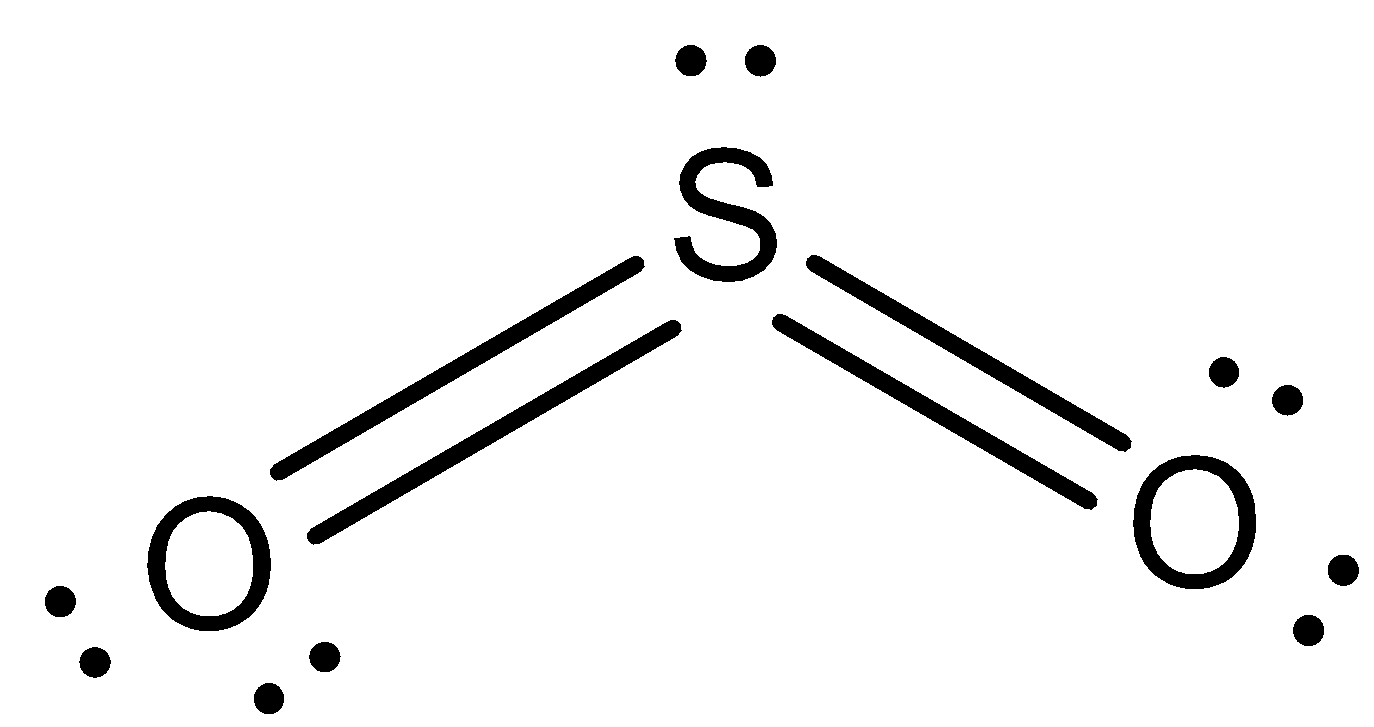
So, the correct answer is “Option B”.
Note: Sulfur dioxide or sulphur dioxide is the chemical compound with the formula SO2. It is a very toxic gas which is the reason for the smell of burnt matches. It is released naturally by volcanic eruptions and is also produced as a by-product of copper extraction and the burning of fossil fuels is also contaminated with a good percentage of sulfur compounds. It is to be remembered that SO2 is not a greenhouse gas, so it does not help in protection of the environment.
Molar mass of the gas is 64.066 gmol−1
IUPAC name is Sulfur dioxide
Melting point: of the gas is 720C
Boiling point of the gas is −100C
