Question
Question: \({\rm{CuS}}{{\rm{O}}_{\rm{4}}}{\rm{.5}}{{\rm{H}}_{\rm{2}}}{\rm{O}}\) is represented as: A. \(\lef...
CuSO4.5H2O is represented as:
A. [Cu(H2O)4]SO4
B. [Cu(H2O)3SO4].2H2O
C. [Cu(H2O)4SO4].H2O
D. [Cu(H2O)5SO4]
Solution
We know that, the chemical name of CuSO4.5H2O compound is copper sulfate pentahydrate. The central metal atom Cu is in +2 oxidation state and it is directly linked to four molecules of water in this complex.
Complete step by step answer:
We know that, CuSO4.5H2O is copper sulfate pentahydrate. It is an inorganic compound. It is a blue colored crystal which is soluble in water. In the structure of CuSO4.5H2O, four molecules of water are directly attached to the central atom copper and one molecule of water is present as water of crystallization. Therefore, we conclude that CuSO4.5H2O is also represented as [Cu(H2O)4SO4].H2O. The structure of this complex is shown below.
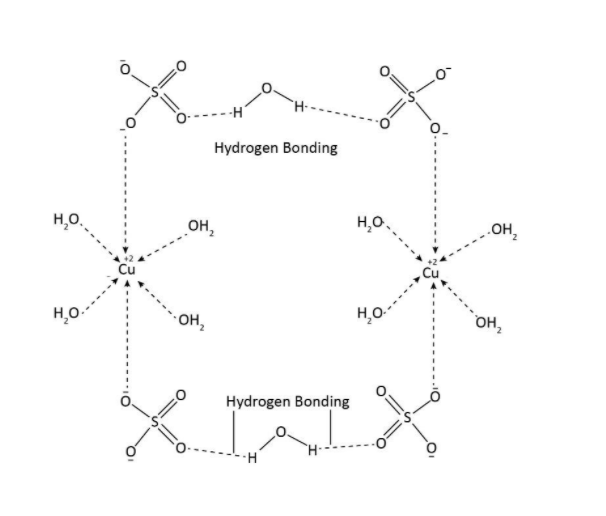
So as we can see, it is clear from the structure that the central metal atom copper is bonded to four water molecules and the remaining fifth water molecule is bonded to sulfate anion through hydrogen bonding (it is already known that the hydrogen bonding is specifically for those atoms which have hydrogen attached to electronegative atom such as oxygen, fluorine and nitrogen).
Hence, we can say that, CuSO4.5H2O is represented as [Cu(H2O)4SO4].H2O.
Thus, the correct option is C.
Note:
As we know that, the inorganic compound CuSO4.5H2O, is a pentahydrate. There are other common names for this compound, which consists of bluestone, blue vitriol, roman vitriol, etc. This complex on dissolution in water gives an aqua complex having chemical formula [Cu(H2O)6]2+. It has an octahedral geometry. In [Cu(H2O)4SO4].H2O, the centres are interconnected through sulfate ions and thus they form chains.
